Πηγές
Μια επιλεγμένη συλλογή πηγών για τη διάγνωση, τη θεραπεία και τη συνεχή αντιμετώπιση ατόμων με πνευμονική νόσο από μη φυματικά μυκοβακτηρίδια (NTM-PD) καθώς και υποκείμενα νοσήματα που αποτελούν παράγοντες κινδύνου.
Στην παρούσα ενότητα, μια βιβλιοθήκη πηγών που αναπτύσσεται συνεχώς και περιλαμβάνει βίντεο, διαδικτυακά σεμινάρια, podcast και άρθρα, προσφέρει αναλυτικές πληροφορίες για τη νόσο NTM-PD και συγκεκριμένα είδη NTM, καθώς και στοιχεία που θα σας βοηθήσουν να αναγνωρίσετε τους ασθενείς σε κίνδυνο για NTM και οδηγίες για το τι πρέπει να κάνετε στην περίπτωση διάγνωσης NTM.

Άρθρο
Κατανοώντας τους παράγοντες κινδύνου για NTM-PD
Read time: 69 mins
Οι κατευθυντήριες οδηγίες ERS/ATS/ESCMID/IDSA για τη NTM-PD 2020 παρουσιάζουν πώς γίνεται η διάγνωση ενός ασθενή με NTM-PD, με βάση τις αξιολογήσεις των κλινικών, απεικονιστικών και μικροβιολογικών δεδομένων.

Άρθρο
Read time: 16 mins
Επισκόπηση συζητήσεων στο ECCMID 2021 με αντικείμενο τη NTM-PD

Άρθρο
Read time: 5 mins
Η μελέτη CONVERT [NCT02344004] αξιολόγησε την αποτελεσματικότητα και την ασφάλεια της εισπνεόμενης λιποσωμιακής αμικασίνης (ALIS [διάλυμα για εισπνοή με εκνεφωτή ARIKAYCE liposomal 590 mg]) σε ενήλικες ασθενείς με NTM-PD ανθεκτική στη θεραπεία

Άρθρο
Read time: 9 mins
Επισκόπηση κλινικών δοκιμών για την NTM-PD οι οποίες είναι σε εξέλιξη ή ολοκληρώθηκαν πρόσφατα

Άρθρο
Επίδραση των μη φυματικών μυκοβακτηριδίων σε ασθενείς με παράγοντες κινδύνου
Read time: 5 mins
Τα μη φυματικά μυκοβακτηρίδια (NTM) μπορούν να προκαλέσουν σοβαρή πνευμονοπάθεια στους ασθενείς με παράγοντες κινδύνου, έχοντας σημαντικό αντίκτυπο στην ποιότητα ζωής τη νοσηρότητα και τη θνησιμότητα και επιταχύνοντας την προοδευτική εξέλιξη της ασθένειας

Άρθρο
Read time: 5 mins
Η αντιμετώπιση της πνευμονικής νόσου από μη φυματικά μυκοβακτηρίδια (NTM-PD) με αντιμικροβιακούς παράγοντες προσφέρει τη δυνατότητα της ίασης. Σε ασθενείς που πληρούν τα κλινικά, απεικονιστικά και μικροβιολογικά διαγνωστικά κριτήρια για NTM-PD.

Άρθρο
Read time: 7 mins
Η πνευμονική νόσος που οφείλεται στο σύμπλεγμα άτυπων μυκοβακτηριδίων MAC (MAC-PD) παρουσιάζει προβλήματα στη διάγνωση, καθώς τα συμπτώματα είναι παρόμοια με εκείνα υποκείμενων πνευμονικών νοσημάτων.

Άρθρο
Κατανοώντας τις βέλτιστες πρακτικές στην πνευμονική νόσο από το σύμπλεγμα MAC (MAC-PD)
Read time: 10 mins
Η θεραπεία της πνευμονικής νόσου από μη φυματικά μυκοβακτηρίδια (NTM-PD) ποικίλλει ανάλογα με το είδος των μυκοβακτηριδίων, την έκταση της νόσου, τα αποτελέσματα από τη δοκιμή ευαισθησίας σε αντιφυματικά φάρμακα και υποκείμενες συννοσηρότητες.

Άρθρο
Read time: 8 mins
Η πνευμονική νόσος από μη φυματικά μυκοβακτηρίδια (NTM-PD) εμφανίζεται όλο και συχνότερα και μπορεί να οδηγήσει στον θάνατο. Οι διεθνείς κατευθυντήριες οδηγίες του 2020 παρέχουν συστάσεις σχετικά με τα τέσσερα συχνότερα παθογόνα είδη NTM.

Άρθρο
Διαχείριση ασθενών στη διάρκεια μιας πανδημίας
Read time: 5 mins
Η πανδημία COVID-19 έφερε την τηλεϊατρική στο προσκήνιο του τομέα υγείας. Ο τομέας που σχετίζεται με την παρακολούθηση της πνευμονικής νόσου από μη φυματικά μυκοβακτηρίδια (NTM-PD) είναι ένας από τους πολλούς που ασπάστηκε αυτήν την αλλαγή.

Βίντεο
Κατευθυντήριες οδηγίες ATS/ERS/ESCMID/IDSA για τη NTM-PD 2020 – Παρουσίαση ειδικών
Stefano Aliberti, Christoph Lange, Eva Polverino, Nicolas Veziris, Charles Haworth and Jakko van Ingen
Η πνευμονική νόσος από μη φυματικά μυκοβακτηρίδια (NTM-PD) εμφανίζεται όλο και συχνότερα και μπορεί να οδηγήσει στον θάνατο. Οι διεθνείς κατευθυντήριες οδηγίες του 2020 παρέχουν συστάσεις σχετικά με τα τέσσερα συχνότερα λοιμογόνα είδη NTM.

Βίντεο
Ποιοι είναι οι ασθενείς που διατρέχουν κίνδυνο για MAC-PD
Οι υποκείμενες πνευμονικές παθήσεις ή η ανοσοκαταστολή αυξάνουν σημαντικά τον κίνδυνο νόσησης από MAC-PD. Δείτε σε αυτό το βίντεο τους ειδικούς να αναλύουν ποιοι είναι οι ασθενείς με παράγοντες κινδύνου και ποια τα ενδεικτικά συμπτώματα.

Βίντεο
Η απόφαση για το ποια είναι η κατάλληλη στιγμή για την έναρξη της θεραπείας στη MAC-PD εξαρτάται από πολλούς παράγοντες. Σε αυτό το βίντεο, ειδικοί διερευνούν το σκεπτικό και το χρονικό πλαίσιο για την έναρξη της θεραπείας.

Βίντεο
Συνεχής διαχείριση ασθενών με MAC-PD
Δείτε και ακούστε διεθνείς ειδικούς να διερευνούν τα βασικά στοιχεία στην εφαρμοζόμενη θεραπευτική μεταχείριση έως τη μετατροπή της καλλιέργειας και μετά από αυτήν.

Βίντεο
Τι πρέπει να γίνει στην περίπτωση αποτυχίας της θεραπείας
Σε αυτό το βίντεο, ειδικοί διερευνούν τις επιλογές που έχουν οι ιατροί για τη MAC-PD όταν η θεραπεία αποτυγχάνει.

Βίντεο
Μικροβιολογική κατάσταση κατά τη διάγνωση και τη θεραπεία της MAC-PD
Σε αυτό το βίντεο, ειδικοί μοιράζονται τις σκέψεις τους για τον ρόλο των μικροβιολογικών εξετάσεων στη διάγνωση, στον καθορισμό αποτελεσματικών θεραπευτικών στρατηγικών και στην καθημερινή παρακολούθηση με στόχο την αρνητικοποίηση της καλλιέργειας.

Βίντεο
Κατευθυντήριες οδηγίες για τη NTM-PD – Βασικές συστάσεις
Σε αυτό το βίντεο, οι Ευρωπαίοι εμπειρογνώμονες παρέχουν τις γνώσεις τους σχετικά με τις οδηγίες ATS/ERS/ESCMID/IDSA 2020 για το NTM-PD, με έμφαση στο MAC-PD.

Βίντεο
www.RethinkNTM – Ποιος, γιατί και πότε; ERS 2020
Παρακολουθήστε το συνέδριο ERS 2020 με χορηγό την Insmed και ενημερωθείτε για τον ασθενή με παράγοντες κινδύνου για NTM-PD, τις προκλήσεις στη διαχείριση της NTM-PD και τις συστάσεις των κατευθυντήριων οδηγιών ATS/ERS/ESCMID/IDSA 2020.

Βίντεο
Με το βλέμμα στο μέλλον - το μεταβαλλόμενο τοπίο της πνευμονικής λοίμωξης από MAC - WBNC 2020
Παρακολουθήστε το συνέδριο WBNC 2020 με χορηγό την Insmed και ενημερωθείτε για τις εξελίξεις στη διαχείριση της NTM-PD, τις συστάσεις των κατευθυντήριων οδηγιών ATS/ERS/ESCMID/IDSA 2020 και τη χρήση της ALIS σε κλινικό περιβάλλον.

Συλλογή διαφανειών
Συλλογή διαφανειών με κατευθυντήριες οδηγίες ATS/ERS/ESCMID/IDSA για τη NTM-PD
Σε αυτή τη σύντομη συλλογή διαφανειών θα βρείτε μια επισκόπηση των οδηγιών ATS/ERS/ESCMID/IDSA 2020.

αφίσα
Σύντομος οδηγός με κατευθυντήριες οδηγίες ATS/ERS/ESCMID/IDSA για τη NTM-PD
Βρείτε στον σύντομο οδηγό τσέπης τις συστάσεις των κατευθυντήριων οδηγιών ATS/ERS/ESCMID/IDSA 2020 για την NTM-PD
Managing patients during a pandemic
The COVID-19 pandemic has brought telemedicine to the forefront of healthcare. The monitoring of non-tuberculous mycobacterial pulmonary disease (NTM-PD) is one of the many aspects of healthcare to adopt this change, with many patients trading in-person consultations with clinicians for video consultations, as well as sending sputum samples through the post for analysis. As a result of its success during the pandemic, many believe that soon telemedicine will form an integral part of healthcare.
The COVID-19 pandemic has brought about many changes to everyday life. Perhaps one of the more beneficial changes to arise during the pandemic is the switch to telemedicine. The idea of telemedicine has been around for a while, but the pandemic and the need for social distancing measures have provided the momentum to adopt this approach into routine practice.1,2
Telemedicine is the use of digital communications to provide remote support for patients to manage aspects of their health.1 The need to limit contact between individuals to reduce the risk of transmission during the pandemic has led to a rapid uptake of telemedicine around the world to help keep both patients and healthcare professionals (HCPs) safe while allowing continuation of routine care.2 Examples include a shift toward telephone and video consultations as well as patients providing laboratory samples through the post, which is currently the case for many patients with NTM-PD.
The management of patients with NTM during the pandemic represents a unique challenge because of the need for regular monitoring of these patients. During a symposium, ‘Looking ahead – the changing landscape of MAC lung infection’, at the 4th World Bronchiectasis & NTM Conference (WBNC) 2020, experts described how the management of NTM-PD has changed in their practices in response to the COVID-19 pandemic.3 Eva Polverino (Vall D’Hebron University Hospital, Spain) explained that following an initial shock response in early 2020 as the pandemic took hold, there was a drive to maintain effective management of patients with NTM-PD during the pandemic, and so telemedicine was introduced. For her, it was important to decide with patients which tests and which visits were a priority, and which could be delayed. Stefano Aliberti (University of Milan, Italy) also emphasised the importance of the new international NTM management guidelines,4 which he believes have been one of the major steps forward in the management of NTM-PD, particularly during the pandemic. Not only have the guidelines helped in the management of NTM-PD, but also in raising awareness of the disease.
Patients with NTM-PD who are receiving treatment should typically be monitored with monthly sputum samples to assess treatment response. During the pandemic, many clinicians have found it more difficult to monitor their patients with NTM-PD, as patients cannot frequently travel to hospitals to deliver sputum samples. However, sputum samples can be sent through the post to enable effective monitoring. Patients are sent tubes for sputum collection that they then post to a laboratory for testing. Evidence has shown that sputum is stable at room temperature for at least 7 days,5 samples are good enough quality to be used for culture and there is no additional burden on laboratories to process these samples.
Telemedicine may be an appropriate option for those patients who are already diagnosed and in receipt of treatment but may be more difficult in those patients who develop symptoms of NTM-PD that may be vague or like those of their underlying lung conditions presenting virtually to their respiratory clinic or primary care physician. It is known that patients with NTM-PD who do not receive treatment, outcomes can be poor, and their health-related quality of life adversely affected,6,7 so encouraging patients with symptoms of NTM-PD to discuss these with their physician and for physicians to have an index for suspicion to test patients for NTM-PD is required; the role of telemedicine in these patients is yet to be fully explored and exploited.
Instead of postal sputum samples, some clinics such as the Policlinico of Milan attended by Stefano Aliberti have created systems with specific time-slots or local collection points where patients can regularly drop off their sputum samples for evaluation. In some vulnerable patients, sputum samples can be collected every 3 months, and it is up to the HCP and their patient to decide what is best.
The opinion from experts is that the management of patients diagnosed with NTM-PD who are already receiving antibiotic therapy during the pandemic has remained effective in most cases. This has been achieved through the use of community hubs for sputum collection or submission of sputum by mail to expert centres and development of COVID-19 free pathways within hospitals or COVID-19-free sites/units within hospitals. However, it is known that across healthcare in general there has been a reduction in the number of patients with symptoms presenting to healthcare professionals and delays in referred investigations8,9 but the impact of this on care for NTM-PD patients not yet diagnosed is unknown.
Indeed, in one survey among respiratory physicians, 95% of respiratory consultants said that the pandemic had a negative impact on respiratory care of non-COVID patients, and 88% said that waiting lists for routine respiratory care have increased, in some cases by more than 50%.10
The shift to telemedicine has facilitated patient and HCP safety during the pandemic and has had the additional benefit of improving patient outcomes in some cases.1,2 It has allowed continuation of routine care from the safety of the home, reducing the need for non-essential trips to the hospital.1 By increasing the ability for patients to self-manage their condition from home, telemedicine may also have helped minimise the load on healthcare systems, and some clinicians have been able to significantly increase the number of patients they can consult in a day.2,11 Moving forward in a post-pandemic world, telemedicine may have gathered enough traction to be maintained in the future as an integral part of healthcare.1 Taking experiences from the pandemic, telemedicine could pave the way for the development of more advanced healthcare solutions including using technologies such as wearable devices and smart phones to support routine care, transforming healthcare in the long term.2
Βιβλιογραφία:
- Ahmed S, et al. BMJ Innov 2020;6:252–4.
- Blandford A, et al. Lancet Global Health 2020;8(11):e1364–e1365.
- Looking ahead – the changing landscape of MAC lung infection, Insmed, 4th World Bronchiectasis & NTM Conference, 16–19 December 2020. http://www.world-bronchiectasis-conference.org/wp-content/uploads/2020/12/WBNC2020_Final_Programme_V1_2.pdf [Accessed March 2021].
- Daley CL, et al. Eur Respir J 2020;56:2000535.
- Pennings LJ, et al. Diagn Microbiol Infect Dis 2018;92:309–10.
- Mehta M, et al. Respir Med 2011;105:1718–25.
- Yeung MY, et al. Respirology 2016;21:1015–25.
- Bower E. GP 5 May 2020. https://www.gponline.com/urgent-referrals-rejected-one-three-gps-during-covid-19-outbreak/article/1682282 [Accessed March 2021].
- British Medical Association. The hidden impact of COVID-19 on patient care the NHS in England. https://www.bma.org.uk/media/2840/the-hidden-impact-of-covid_web-pdf.pdf [Accessed March 2021].
- ITS Annual Scientific Meeting Press Release, Dec 3rd 2020. https://irishthoracicsociety.com/2020/12/its-annual-scientific-meeting-press-release-dec-3rd-2020/ [Accessed March 2021].
- Webster P. Lancet 2020;396(10231):1180–1.
 Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
CONVERT Study: The efficacy, sustainability and long-term safety of ALIS for patients with treatment-refractory MAC-PD
The CONVERT study [NCT02344004] evaluated the efficacy and safety of amikacin liposomal inhalation suspension (ALIS [ARIKAYCE liposomal 590 mg nebuliser dispersion]) in adult patients with treatment-refractory non-tuberculous mycobacterial pulmonary disease (NTM-PD) caused by Mycobacterium avium complex (MAC) in addition to oral guideline-based therapy (GBT) compared with oral GBT alone. ALIS plus oral GBT demonstrated high rates of culture conversion in 6-month data published in 2018 compared with GBT alone (29% vs 9%) and a follow-up study demonstrated culture conversions were often sustained and durable, and there were no new safety signals emerged with long-term use of ALIS.
MAC-PD is a difficult-to-treat pulmonary infection. When initial oral GBT fails, outcomes are poor and options are limited.1,2 ALIS is a novel amikacin formulation that penetrates alveolar macrophages and biofilms while limiting systemic exposure.3–5 ALIS is currently recommended by guidelines for patients with MAC-PD who fail to achieve culture conversion after at least 6 months of oral GBT in combination with oral GBT.6 ALIS has been previously tested in a Phase II study of treatment-refractory non-tuberculous mycobacterial pulmonary disease (NTM-PD) where the addition of ALIS to standard oral GBT achieved higher rates on negative sputum cultures compared with oral GBT alone.7
CONVERT was a prospective, open-label, randomised trial that evaluated the efficacy and safety of daily ALIS in addition to oral GBT in patients with refractory MAC-PD compared with oral GBT alone. A total of 336 patients with amikacin-susceptible MAC-PD and MAC-positive sputum cultures after receiving at least 6 months of oral GBT were randomised at a 2:1 ratio to receive either ALIS plus oral GBT or oral GBT alone. The primary endpoint was the proportion of patients achieving culture conversion, which was achieved if patients had three consecutive monthly MAC-negative sputum cultures by Month 6 of the study. The study was conducted in 127 centres across 18 countries in North America, Asia-Pacific and Europe. Patients were mostly female (69.3%) with a mean age of 64.7 years and many patients had underlying bronchiectasis (62.5%), chronic obstructive pulmonary disease (14.3%) or both (11.9%). The majority of patients (89.9%) were receiving GBT at enrolment, with the remainder off treatment for 3–12 months. Most patients (69.3%) were on a three-drug regimen at baseline, with 54.9% on regimens which included a macrolide, ethambutol and a rifamycin.8
Initial results of the trial demonstrated the efficacy of ALIS in addition to oral GBT in achieving culture conversion by Month 6.8 Patients treated with ALIS in addition to oral GBT were almost four times as likely to achieved culture conversion by Month 6,8 with 29% (n=65/224) of patients on ALIS plus oral GBT achieving culture conversion compared with only 8.9% (n=10/112) on oral GBT alone (P<0.0001).8,9
In a follow-up study published in 2021, patients who achieved culture conversion by Month 6 continued treatment for an additional 12 months, followed by off-treatment observation in order to assess the sustainability and durability of culture conversion. Following 12 months of post-conversion treatment, 63.1% (n= 41/65) of converters in the ALIS plus oral GBT arm and 30.0% (n=3/10) in the oral GBT alone arm achieved sustained conversion (P=0.0644). In the intention-to-treat population, which includes patients who did not culture convert, 18.3% (n=41/65) of patients in the ALIS plus oral GBT arm achieved sustained culture conversion compared with only 2.7% (n=3/10) in the oral GBT alone arm (P<0.0001). Three months following end of treatment, 55.4% (n=36/65) of ALIS plus oral GBT culture-converted patients also achieved durable culture conversion whereas no patients on oral GBT alone achieved durable culture conversion (P=0.0017). In the intention-to-treat population, 16.1% (n=36/224) of all patients on ALIS plus oral GBT achieved durable culture conversion versus no patients treated with oral GBT alone (P<0.0001).9
Re-emergence of a MAC strain with an identical genotype to the strain identified on initiation of treatment may indicate relapse, particularly if this occurs within the first 8 months of treatment. At the end of treatment, only 7.7% (n=5/65) of patients on ALIS plus oral GBT had relapsed compared with 30% (n=3/10) of patients on oral GBT alone. In contrast, recurrence of MAC after 8 months of treatment may indicate reinfection, in which MAC has been reacquired from the environment, and is treated as a new MAC infection. In total, 4.6% (n=3/65) of patients on ALIS plus oral GBT were reinfected with MAC, compared with 10% (n=1/10) of patients on oral GBT alone.9
Treatment emergent adverse events (TEAEs) occurred mainly in the first 8 months of treatment and were mainly respiratory. Respiratory TEAEs were reported more frequently in the ALIS plus oral GBT arm and included dysphonia (61.5%), cough (41.5%), dyspnoea (21.5%), and haemoptysis (20.0%). Nephrotoxicity TEAEs were balanced between the two arms (n=2 in both arms) and ototoxicity-related TEAEs in the ALIS plus GBT arm were primarily tinnitus (10.8%) and dizziness (7.7%). Only four patients discontinued treatment because of TEAEs in the ALIS plus oral GBT converter arm. Three patients who achieved culture conversion died, all in the oral GBT alone arm.9
The CONVERT study showed that the addition of ALIS to oral GBT significantly increased the likelihood of culture conversion by Month 6 compared with oral GBT alone, providing the first evidence in a randomised trial in addition to the Phase II study of efficacy against treatment-refractory MAC-PD (Figure 1).8 In addition, culture conversion in patients treated with ALIS in addition to oral GBT was generally sustained and durable with low risk of relapse (Figure 1).9 Long-term exposure to ALIS did not present any new safety concerns, with most TEAEs consistent with administration of an inhaled add-on antibiotic and generally occurring in the first 8 months of treatment.9 Overall, these results highlight the clinical utility of ALIS in the management of patients with refractory MAC-PD.
Figure 1. Proportion of patients achieving culture conversion by the first month of conversion.8,9
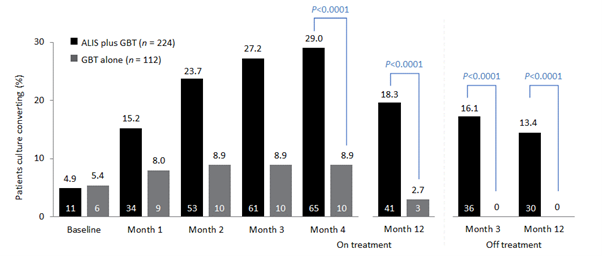
Month 4 was the last time point at which the first of three negative sputum cultures could be achieved for a patient to be considered a converter at Month 6.
ALIS, amikacin liposomal inhalation suspension; GBT, guideline-based therapy.
Βιβλιογραφία:
- Griffith DE, Aksamit TR. Therapy of refractory nontuberculous mycobacterial lung disease. Curr Opin Infect Dis 2012;25:218–27.
- Jo K-W, Kim S, Lee JY, Lee SD, Kim WS, Kim DS, et al. Treatment outcomes of refractory MAC pulmonary disease treated with drugs with unclear efficacy. J Infect Chemother 2014;20:602–6.
- Rose SJ, Neville ME, Gupta R, Bermudez LE. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS ONE 2014:9:e108703.
- Malinin V, Neville M, Eagle G, Gupta R, Perkins WR. Pulmonary deposition and elimination of liposomal amikacin for inhalation and effect on macrophage function after administration in rats. Antimicrob Agents Chemother 2016;60:6540–9.
- Zhang J, Leifer F, Rose S, Chun DY, Thaisz J, Herr T, et al. amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol 2018;9:915.
- Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020;56:2000535.
- Olivier KN, Griffith DE, Eagle G, McGinnis JP 2nd, Micioni L, Liu K, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 2017;195:814–23.
- Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium aviumcomplex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med 2018;198:1559–69.
- Griffith DE, Thomson R, Flume PA, Aksamit TR, Field SK, Addrizzo-Harris DJ, et al. Amikacin liposome inhalation suspension for refractory Mycobacterium avium complex lung disease: sustainability and durability of culture conversion and safety of long-term exposure. Chest 2021; https://doi.org/10.1016/j.chest.2021.03.070
![]()
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
NTM-PD at ECCMID 2021
Non-tuberculous mycobacterial (NTM) infection and NTM pulmonary disease (NTM-PD) are rare diseases and have largely been overlooked in the past. It was welcome to see at the 2021 ECCMID annual congress that NTM and NTM infection/NTM-PD are emerging from the shadows with 3 symposia and more than 20 abstracts directly related to the species identification, diagnosis or treatment of NTM infection and NTM-PD. An overview of the most relevant information presented pertaining to NTM-PD specifically and NTM infection as it might influence our thinking for NTM-PD is provided here and presents an exciting snapshot into emerging scientific and clinical efforts to combat this disease.
For the first time NTM infection and NTM-PD has had a noticeable presence within a highly visible European microbiological congress. A wealth of scientific and clinical research is emerging to tackle the challenges of NTM-PD with the aim, in the future, of improving the clinical outlook for patients.
New recommendations for treating NTM-PD – the 2020 guidelines
In the “Meet The Expert session” ‘Nontuberculous mycobacterial pulmonary disease – the new ATS/ERS/IDSA/ESCMID guideline’, Dr Jakko van Ingen and Professor Claire Andrejak discussed the content to orient participants on new recommendations and answer questions from the audience.
For the first time guidelines for NTM-PD are international providing consistent evidence-based recommendations based on 22 PICO questions with graded evidence of systematic literature review.1 The limitation of the guidelines is the focus only on 4 key mycobacterial species MAC, M. kansasii, M. abscessus, M. xenopi.1 Importantly the guidelines now cover microbiological diagnostics with clear messages to obtain ≥3 respiratory samples each obtained >1 week apart, a recommendation for full speciation of the organism identified so that the clinical virulence of the infecting organisms can be determined, and clear guidelines to undertake susceptibility testing once species are identified. Dr van Ingen recommended to test and report microbiological susceptibility as per CLSI M24/M62 guidelines in broth microdilution and, if not available in regional labs, samples should be sent to reference centres. Diagnostic criteria in 2020 remain unchanged from 20072 and focus on clinical symptoms, radiological evidence and microbiological evidence of 2 positive culture of the same subspecies in order to exclude errors from a single sample.
New recommendations exhort clinicians to start treatment in patients with positive acid-fast bacilli sputum smears as this is suggestive of a high bacterial load, or if there is radiological evidence of cavitary lung disease suggestive of progressive disease.1 Possible reasons to wait to initiate treatment besides mild disease include assessing the readiness of the patient to begin an arduous treatment journey of 12 months or more, understanding of the drug susceptibility of the species identified and potential for recurrent infection.
In MAC-PD macrolides form the backbone of treatment and the 2020 guidelines suggest that azithromycin over clarithromycin should be considered, and in the absence of additional clinical data, macrolides plus ethambutol and rifampicin should be used as a 3-drug regimen;1 although Professor Andrejak outlined that studies of 2 drug regimens in MAC-PD are underway (NCT03672630). In patients with severe disease parenteral amikacin or streptomycin should be considered in the early treatment period. For those patients not culture converting by 6 months, the guidelines newly recommend the addition of Amikacin Liposomal Inhalation Suspension (ALIS) based on Phase 3 clinical data from the CONVERT study.1,3
In M. xenopi no correlation between drug susceptibility and clinical outcomes exists and so susceptibility testing is not recommended. With respect to treatment moxifloxacin or clarithromycin can be used and should be included in a treatment regimen of at least 3 drugs.1 Professor Andrejak suggested the possibility to use parenteral amikacin in M. xenopi PD, and an investigator-led study in France to explore the utility of ALIS in M. xenopi is due to start.
In M. kansasii treatment recommendations are to use 3 drug regimens of rifampicin, ethambutol and isoniazid or azithromycin for 12 months; there is no role for aminoglycosides.1 The guideline recommends susceptibility testing at baseline for rifampicin and clarithromycin, particularly given the increase in macrolide resistance. In instances of rifampicin resistance or patients intolerant to rifampicin then fluoroquinolones can be used, but this applies only to M. kansasii and not to other species.1 In patients with mild nodular disease with a macrolide-based regimen a thrice weekly dosing regimen is possible, but all regimens should be dosed for 12 months.
M. abscessus bacterial complex (MABC) -PD is one of the most difficult mycobacterial infections to treat1 but the speakers, both authors of the guidelines noted that current recommendations are relatively weak for this species due to lack of evidence. It was stressed that sub-speciation of M. abscessus is essential as M. abscessus subsp. massiliense is macrolide susceptible whilst M. abscessus subsp. abscessus may be susceptible but prone to inducible resistance. At this time the recommendation is to work closely with an expert centre for NTM-PD and treatment should focus on at least 3 drugs including amikacin, imipenem, macrolides, tigecycline and clofazimine. Use of macrolides depends on susceptibility and should not be used in cases of mutational resistance. The duration of treatment post-culture conversion is as yet unknown and the composition of long-term regimens has not been determined. Dr van Ingen highlighted the need for full clinical trials in M. abscessus rather than case series as are currently available – the medical unmet need in these patients is high and further data on appropriate therapy is needed.
Professor Andrejak counselled that continued monitoring especially microbiological evaluation of sputum every 1–2 months on treatment is essential to determine response to therapy. Similarly, monitoring for adverse events is essential and should focus on liver function tests, audiograms, ECG and so on dependent on the antimicrobials included in the treatment regimen.
Within this session, an overview of new drugs in the pipeline were presented and this demonstrates an unprecedented era of focus and development for NTM-PD. These include minocycline, tedizolid, clofazimine and ALIS that are being evaluated in the laboratory, dynamic models such as hollow fibre models and early human Phase 1/Phase 2 studies.
Meeting the challenges of NTM organisms and NTM-PD
The symposium ‘NTM-PD: do we need to rethink its management’ chaired by Dr van Ingen and Dr Daniela Cirillo (sponsored by Insmed) explored the challenges mycobacterial pulmonary infection present, that requires a new way of thinking for management.
NTM present a particular challenge to treatment because of their cellular physiology including hydrophobic, thick cell walls and their ability to sequester in intracellular spaces including phagocytic cells and biofilms.4–7 Professor Matteo Bassetti presented an overview of sequestration into intracellular spaces and how NTM species, such as M. avium, manipulate normal macrophage processes to reduce phagosome-lysosome fusion, up-regulate genes to facilitate MAC replication and reduce macrophage function so that macrophage apoptosis is controlled enabling effective release of MAC bacteria into the lung environment and infection of neighbouring macrophages so driving a cycle of infection.8–10 Similarly, incorporation of NTM organisms into biofilms presents a physical challenge to the host and to antimicrobial entry and biofilms persist following initiation of phagocyte apoptosis to arrest normal biofilm breakdown mechanisms.11
The problem of the mycobacterial physiology is also coupled with ubiquitous distribution in the environment as presented later in the symposium by Professor Veziris.12,13 NTM-PD is largely initiated by inhalation of organisms in patients with underlying risk factors or may be aspirated from the gastrointestinal tract. Once in the lung NTM can evade antimicrobial action as lung penetration of many systemically administered antibiotics is limited14 requiring high doses to achieve sufficient lung concentrations which may not be possible due to side effects.15 Penetration of many antibiotics into intracellular spaces such as macrophages and biofilms is also poor.14–16
Despite ubiquitous distribution of NTM organisms, exposure does not equate to universal infection. Rather a series of underlying risk factor predispose the tipping point from exposure to infection including underlying lung conditions and some patient morphological characteristics.17 Similarly, diagnosis of NTM-PD or MAC-PD in a patient may not lead to immediate treatment as there are factors of spontaneous culture conversion,18 patient comorbidities and patient wishes to consider.
Aerosolised inhaled antibiotics may address the problem of lung penetration and may reduce selection pressure for multi-drug resistant organisms but is unable to address the issue of macrophage or biofilm penetration providing a rationale for liposomal encapsulation. Liposomes provide an opportunity to penetrate cell membranes, to improve pharmacokinetics of encapsulated antibiotics and potentially reduce systemic toxicity.19 ALIS) licensed in Europe as ARIKAYCE® liposomal 590 mg nebuliser dispersion, is the first inhaled liposome encapsulated antibiotic to be approved and is indicated for use in adult patients with MAC-PD who have limited treatment options and do not have cystic fibrosis, in consideration of official guidance on the appropriate use of antibacterial agents.20 Early studies have demonstrated effective deposition in the lung post-inhalation that persists over 24 hours, and effective penetration in an in vitro study of both MAC infected macrophages and biofilms.21,22
For MAC-PD treatment is lengthy and relies on a macrolide backbone of azithromycin plus ethambutol and rifampicin for at least 6 months to secure culture conversion and then 12 months beyond.1 Professor Veziris presented data to support a new recommendation in the guideline, that of prescribing ALIS to patients with MAC-PD who fail to culture convert by 6 months. A Phase 3 study has demonstrated that using ALIS in patients who have failed oral guideline-based therapy (GBT), many of whom had refractory disease for many years, provides culture conversion in 29% of patients compared with GBT alone 8.9% (p<0.0001);3,23 and it is in these data that guidelines have been revised for patients with MAC-PD (Figure 1).1 Professor Veziris presented further data from ALIS from the long-term follow-up phase of the Phase 3 study which demonstrates that culture conversion is durable while patients are on ALIS plus GBT therapy and is sustained for 3 months or more once all antimicrobial therapy is removed.23
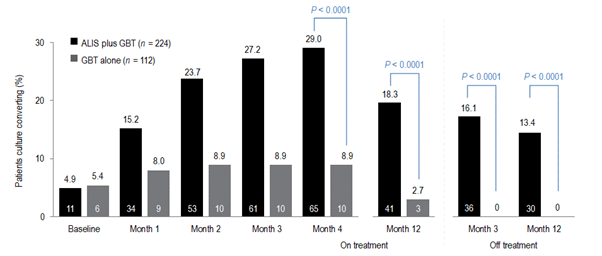
Figure 1. Proportion of patients achieving or maintaining culture conversion
Month 4 was the last time point at which the first of three negative sputum cultures could be achieved for a patient to be considered a convertor at month 6.
GBT, guideline-based therapy.
Emerging technologies and treatments in NTM-PD
The symposium ‘What’s new in mycobacterial disease’, chaired by Professor Florian Maurer and Professor Thomas Schön, explored a range of new developments in NTM-PD and NTM infection. The symposium included two presentations that have the future potential to impact clinical management, one by Dr van Ingen to explore a biomarker to predict treatment success in NTM-PD and one about the potential activity of pentamidine in MAC and M. abscessus from Professor Jelmer Raaijmakers.
Biomarkers in NTM-PD have potential to provide insight into when is the best time to treat patients with disease, the impact of treatment and determining treatment success. Dr van Ingen presented data of a biomarker that can aid prediction of culture conversion in patients once treatment is initiated. Treatment regimens for NTM-PD are often hampered by a limited evidence base and a poor rate of culture conversion despite aggressive treatment.24–26 Time to positivity for MAC organisms in sputum culture was presented as a possible tool to predict patients who will respond to treatment that could be useful in clinical practice and in clinical trials to evaluate new therapies.
The Mycobacterium Growth Indicator Tube (MGIT™) is an automated liquid culture system.27 Using sputa from 49 patients the time to positivity (TTP) in the MGIT system was explored as a biomarker for treatment response. All patients had macrolide-sensitive MAC-PD and TTP was correlated with actual clinical outcomes of conversion, defined as 2 consecutive negative cultures collected ≥4 weeks apart. Mean baseline TTP was higher in patients who culture converted than those who did not (7.68 ± 4.64 vs 4.87 ± 2.20 days, p=0.031), and TTP was also significantly different for patients with nodular-bronchiectatic disease and those with fibrocavitary disease (8.86 ± 5.62 vs 5.29 ± 1.65 days, p=0.010). Differences in TTP increased over time so that, at 3 months, TTP for those converting was 36.38 ± 12.30 days compared with 9.75 ± 5.19 days in non-convertors (p<0.001). These data suggest that MGIT TTP obtained at baseline and at 3 months provides a prediction of culture conversion for MAC-PD. However, Dr van Ingen was keen to outline that time to positivity is predictive of culture conversion only and cannot predict treatment and patient outcomes. However, the use of an early and easily available biomarker that can predict patients who are most likely to convert with therapy can be extremely helpful in planning treatment strategies for individual patients.
New therapies in NTM-PD
Novel treatment approaches were also a focus of NTM abstracts. One by Kan et al.28 suggested that the ligase PafA in the pup-proteosome system (PPS) which is essential for maintaining bacterial persistence in macrophages might provide a potential drug target for patients with persistent intracellular NTM infection. Using proteomic analysis three PafA inhibitors were identified and demonstrated reductions in intracellular mycobacterium in vitro in macrophages. The inhibitors discovered require more investigation but provide an interesting potential adjunct for treating mycobacterial infections such as NTM-PD.
ALIS as a liposomal formulation has been demonstrated in vitro to penetrate macrophages and biofilms where MAC organisms typically sequester to evade host defences and antimicrobial therapy.22 The study by Le Moigne et al.29 explored the ability of ALIS to penetrate phagocytic cells where MABC organisms reside. In this study, access to intracellular mycobacteria was explored using confocal microscopy to observe potential co-localization of ALIS and MABC in cells including epithelial cells and macrophages, and to explore intracellular antimicrobial activity. Confocal microscopy demonstrated that fluorescently tagged ALIS co-localises with MABC within a range of cells, not just phagocytic ones such as macrophages but also epithelial cells, an effect that was not observed with water soluble amikacin. Within cells ALIS demonstrated intracellular bactericidal at concentrations of 32 and 64 μg/mL at 3- and 5-days post-infection. Together, these data suggest that ALIS provides potential in MABC infection with an ability to reach intracellular spaces and have an antimicrobial action on MABC.
M. kansasii pulmonary disease is a common disease-causing mycobacterium second only to MAC and is associated with a poor outlook – with mortality rates up to 50% in patients co-infected with HIV.30 A study by Munoz-Munoz et al.31 explored the susceptibility profile of beta-lactams. Beta-lactam antibiotics are not typically used for mycobacterial infections due to the presence of constitutive beta-lactamases, but in this study beta-lactams in combination with clavulanate did demonstrate potency against the M. kansasii strain ATCC although less than with guideline-based antimicrobial therapy. Amoxicillin/clavulanate was the most active combination (MIC 8 mg/mL) but carbapenems even in the presence of clavulanate had no activity.
Emerging approaches to treating NTM-PD are the development of new molecules or understanding how older molecules can be repurposed. Professor Raaijmakers presented an interesting study exploring the use of pentamidine.32 Pentamidine is most used as an inhaled antibiotic for the treatment of pneumocystis pneumonia and Professor Raaijmakers presented data of pentamidine in isolate models to understand isolate susceptibility, intracellular penetration and efficacy against isolates in phagocytic cells and efficacy against isolates in an ELF model. In vitro time-kill assays of isolates of M. tuberculosis (n=6), M, abscessus (n=3) and M. avium (n=4) demonstrated greatest efficacy of pentamidine against M. tuberculosis at 0.5 MIC, with efficacy against M. avium at 2 x MIC but very limited response against M. abscessus with regrowth observed even at concentration of 32 x MIC.32 In vivo time-kill assay in human blood mononuclear cells (HBMCs) suggested that pentamidine was comparably effective against M. tuberculosis and M. avium with an ability for intracellular penetration but had very limited activity against M. abscessus. In hollow fibre models that emulate the ELF environment pentamidine plus a GBT regimen of azithromycin, ethambutol and rifampicin reduced bacterial density both extracellularly and intracellularly more than GBT alone, but initial reductions provided by pentamidine were not sustained and within 2–3 weeks bacterial densities in both groups were comparable.32
Improving our understanding of the mechanism of infection of MAC
Dr van Ingen’s group presented an abstract33 that explored the interplay between M. avium phagocytosed into human monocytes and clarithromycin. Post-phagocytosis upregulation in genes related to cytokine signalling and immune activation was evident in macrophages whilst within M. avium genes related to nitrate respiration and coding for M. avium antigens were upregulated. These data highlight that the host environment can greatly influence the efficacy of macrolides such as clarithromycin.
Understanding NTM infection and NTM-PD in areas of high TB
Risk factors for NTM-PD such as underlying lung disease are widely recognised, but an abstract from Cruz et al.34 presented the cases of two patients presenting with symptoms that were assumed to be tubercular given the setting of endemic TB in the country. Only on post-mortem of one patient and sputum testing of the other was NTM infection identified. Whilst these cases are in disseminated NTM infection they provide an insight into countries where TB is endemic to continue to keep NTM infection, NTM-PD and NTM testing front of mind.
As with risk factors, the geographical diversity of NTM species is well known.35 a study from Nigeria,36 a country of endemic TB and high HIV, has explored the species variation across 167 participant sputum samples. In this study in patients with HIV enrolled at a national TB clinic the predominating species was M. intracellulare (45.1%), M. interjectum (16.1%) and M. malmoense (12.9%); M. avium was identified in only 6.5% of samples and 12.9% of samples could not be speciated. These data indicate that NTM-PD infection among people infected with HIV is high, which reflects the similar historical perspective of Western countries before the advent of fully accessible high active anti-retroviral therapy (HAART).
A third study by Fraile Torres et al.37 reminds us that in many parts of the world NTM are overtaking TB as an infecting mycobacterial species. In this study 50,728 sputum samples from 15,931 patients were retrospectively examined for NTM over ten years (2010–2020). Of these isolates 3,328 samples from 1,223 patients were positive for NTM. MABC (M. abscessus subsp. abscessus, M. abscessus subsp. Massiliense, and M. abscessus subsp. bolletii ) was the most common infecting organism and among these patients an equal percentage had the underlying risk factors of NCFBE or CF (34.88%), and a small minority had a history of previous TB.
Translating NTM-PD guidelines into routine practice
Understanding drug susceptibility for any infection is important, and 2020 NTM-PD guidelines recommend specific susceptibility testing depending on the predominating infecting NTM species.1 The antimicrobial susceptibility of a range of slow growing mycobacteria were evaluated by Hunkins et al. in the USA.38 In this study of 10,668 isolates (85.2% of which were respiratory; 5 MAC species, 6 other slow growing NTM) it was noted that susceptibility to macrolides, including clarithromycin was high and consistent among species as was susceptibility to rifabutin except for M. asiaticum and M. simiae where susceptibility was approximately 60% or less. Based on the susceptibility breakpoint for IV amikacin, Susceptibility to amikacin was lower at 76.62% for M. avium and 72.44% for M. intracellulare and given the position of IV amikacin in the treatment of MAC suggests that comprehensive antibiograms may be useful to guide therapy for patients.
In a second study,39 the pattern of susceptibility of MAC isolates was explored. Using MALDI-TOF analysis of 737 strains of MAC in sputum M. avium was the most commonly identified single species (n=351, 47.62%) followed M. intracellulare/M. chimaera (combined n=386, 52.37%). Susceptibility against a range of antibiotics recommended by guidelines1 was explored. It was found that susceptibility of M. avium to clarithromycin was maintained in 95.7% of isolates, but only 3% of isolates were susceptible against ethambutol and even lower for IV amikacin. By contrast, susceptibility for these drugs against M. intracellulare/M. chimaera were better.
A cautionary abstract from India40 demonstrated that disease-driving species differ across the world and that drug susceptibility also varies greatly. In this study, the predominant species causing pulmonary disease were MABC, M. fortuitum and, to a limited extent, M. chelonae. Isolates of M. abscessus were susceptible to clarithromycin but only after extended exposure and there were marked decreases in the susceptibility patterns of isolates to imipenem, cefoxitin and fluoroquinolones compared with those reported from other countries. These data suggest that speciation is vital, and in low- or middle-income countries, infection control measures require improvement. The study authors also suggest that NTM-PD guidelines whilst valuable may not always be applicable across all countries.
In summary
At ECCMID 2021 it was clear that NTM-PD as a rare disease is emerging from the shadows with burgeoning research emerging that gives insight into future diagnostics, prognostics and treatment
Βιβλιογραφία:
- Daley CL, et al. Eur Respir J 2020;56:2000535
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Griffith DE, et al. Am J Respir Crit Care Med 2018;198:1559–69.
- Chakraborty P, Kumar A. Microbiol Cell 2019;6:105–22.
- Sousa S, et al. Int J Mycobacteriol 2015;4:36–43.
- Awuh JA, Flo TH. Cell Mol Life Sci 2017;74:1625–48.
- Ganbat D, et al. BMC Pulm Med 2016;16:19.
- Sturgill-Koszycki S, et al. Science 1994;263:678–81.
- Chiplunkar SS, et al. Future Microbiol 2019;14:293–313
- Lee KI, et al. Scientific Reports 2016
- Rose SJ, Bermudez LE. Infect Immun 2014;82:405–12.
- Lee E-S, et al. J Microbiol Biotechnol 2008;18:1207–15
- Nishiuishi Y et al. CID 2007;45:347-351
- Honeybourne D. Thorax 1994;49:104–6
- Wenzler E, et al. Clin Microbiol Rev 2016;29:581–632
- Greendyke R, Byrd TF. Antimicrob Agents Chemother 2008;52:2019–26
- Prevots DR, Marras TK. Clin Chest Med 2015;36:13–34
- Hwang JA, et al. Eur Repir J 2017;49:1600537.
- Chalmers JD, et al. Eur Respir Rev 2021;30:210010.
- ARIKAYCE liposomal 590 mg nebuliser dispersion. EU Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/arikayce-liposomal-product-information_en.pdf [Accessed September 2021]
- Olivier KN, et al. ATS Congress 2016, San Francisco, CA, USA. Poster A3732.
- Zhang J, et al. Front Microbiol 2018;9:915.
- Griffith DE, et al. Chest 2021;160:831–42Apr 19:S0012-3692(21)00703.
- Kwak N, et al. ERJ 2019; 54:1801991
- Zweijpfenning S, et al. Respir Med 2017;131:220–224.
- Griffith DE, et al. Am J Respir Crit Care Med 2015;192:754–60
- Danho R, et al. ECCMID Congress 2021, virtual. Abstract 02527
- Kan HL, et al. ECCMID Congress 2021, virtual. Abstract 00910.
- Le Moigne V, et al. ECCMID Congress 2021, virtual. Abstract 00866.
- Marras TK, et al. Am J Respir Crit Care Med 2004;170:793–98.
- Munoz-Munoz L, et al. ECCMID Congress 2021, virtual. Abstract 02674
- Raaijmakers J, et al. ECCMID Congress 2021, virtual. Abstract 04116.
- Schildkraut J, et al. ECCMID Congress 2021, virtual. Abstract 03747.
- Cruz MG, et al. ECCMID Congress 2021, virtual. Abstract 00169.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Olayinka A, et al. ECCMID Congress 2021, virtual. Abstract 00942.
- Fraile Torres AM, et al. ECCMID Congress 2021, virtual. Abstract 04043.
- Hunkins J, et al. ECCMID Congress 2021, virtual. Abstract 02793.
- Fernandez-Pittol M, et al. ECCMID Congress 2021, virtual. Abstract 02394.
- Irfana M, et al. ECCMID Congress 2021, virtual. Abstract 02548.
![]()
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Κατανοώντας τους παράγοντες κινδύνου για NTM-PD
Οι κατευθυντήριες οδηγίες ERS/ATS/ESCMID/IDSA για τη NTM-PD 2020 παρουσιάζουν πώς γίνεται η διάγνωση ενός ασθενή με NTM-PD, με βάση τις αξιολογήσεις των κλινικών, απεικονιστικών και μικροβιολογικών δεδομένων (Daley 2020). Ωστόσο, όταν ένας ασθενής επισκέπτεται το νοσοκομείο, τι ακριβώς επάνω του θα παρακινήσει τον κλινικό ιατρό να σκεφτεί τη διεξαγωγή εξετάσεων για NTM;
Αν κατανοήσουμε τους παράγοντες κινδύνου που εμφανίζονται συνήθως στους ασθενείς με NTM-PD, θα έχουμε μια πολύτιμη πληρέστερη εικόνα για τον ασθενή που πιθανώς να επωφεληθεί από τις εξετάσεις για NTM, είτε γιατί θα αποκλειστεί η πιθανότητα της νόσου είτε, στην περίπτωση που όντως υπάρχει λοίμωξη από NTM, γιατί θα καθοριστεί το κατάλληλο σχέδιο δράσης. Στους παράγοντες κινδύνου για νόσηση από NTM-PD περιλαμβάνονται ενδογενείς παράγοντες που σχετίζονται αποκλειστικά με τον ίδιο τον ασθενή (παράγοντες κινδύνου του ξενιστή), περιβαλλοντικοί παράγοντες κινδύνου (έκθεση), ανοσολογικοί παράγοντες κινδύνου, γενετικοί παράγοντες κινδύνου, καθώς και η παρουσία υποκείμενων πνευμονικών παθήσεων ή νοσημάτων (Cowman 2019).
|
Παράγοντες που αυξάνουν την ευαισθησία για NTM-PDΠαράγοντες |
Αυξημένος κίνδυνος ή επιπολασμός της λοίμωξης* |
|
Βρογχεκτασία |
44–187,5 (Prevots 2015, Andrejak 2013) |
|
Πρωθύστερη φυματίωση |
69,0–178,3 (Axon 2019, Andrejak 2013) |
|
Χαμηλός δείκτης BMI |
9,1 (Prevots 2015) |
|
Κυστική ίνωση |
6,6–13,0 (Olivier 2003, Roux 2009) |
|
ΧΑΠ (με λήψη εισπνεόμενων κορτικοστεροειδών) |
29,1 (Andrejak 2013) |
|
ΧΑΠ (χωρίς εισπνεόμενα κορτικοστεροειδή) |
2,0–10,0 (Prevots 2015) |
|
Παραμορφώσεις του θώρακα |
5,4 (Prevots 2015) |
|
Καρκίνος του πνεύμονα |
3,4 (Prevots 2015) |
|
Άσθμα |
2,0–7,8 (Hojo 2012, Andrejak 2013) |
|
Χρήση κορτικοστεροειδών |
1,6–8,0 (Prevots 2015) |
|
ΓΟΠ |
1,5–5,3 (Prevots 2015) |
|
Ρευματοειδής αρθρίτιδα |
1,5–1,9 (Prevots 2015) |
|
Ανοσορυθμιστική/ ανοσοκατασταλτική αγωγή |
1,3–2,2 (Prevots 2015) |
*Λόγος συμπληρωματικών πιθανοτήτων (odds ratio ή OR), σχετικός κίνδυνος ή σχετικός επιπολασμός
Οι παράγοντες που αυξάνουν τον κίνδυνο νόσησης από NTM-PD θα πρέπει να συνυπολογίζονται κατά τη λήψη της απόφασης για το ποιοι ασθενείς θα ελεγχθούν προληπτικά ή θα υποβληθούν σε εξετάσεις για την παρουσία λοίμωξης.
Υποκείμενες πνευμονικές παθήσεις
Βρογχεκτασία
Όπως είναι γνωστό, η βρογχεκτασία συνιστά παράγοντα κινδύνου για τη NTM-PD, αλλά η εκτίμηση της διαβάθμισης του κινδύνου ποικίλλει4,9,10. Η λοίμωξη από τα NTM μπορεί να προκαλέσει βρογχεκτασία, ενώ στους ασθενείς με προϋπάρχουσα βρογχεκτασία μπορεί να διευκολύνει την προοδευτική εξέλιξη της νόσου, καθώς οι βρόγχοι με ανατομικές αλλοιώσεις είναι ευπαθείς σε λοιμώξεις 11. Για τους ασθενείς με βρογχεκτασία, εκτιμάται ότι ο κίνδυνος να νοσήσουν από NTM-PD είναι κατά 44 έως 187,5 φορές μεγαλύτερος3,4, ενώ ο επιπολασμός της NTM-PD μεταξύ των ασθενών με βρογχεκτασία εκτιμήθηκε περίπου στο 9,3%.12
Η βρογχεκτασία συσχετίζεται με τη NTM-PD σε γυναίκες ασθενείς, με χαμηλή μάζα λίπους, ακόμα και μετά τις ρυθμίσεις για τον δείκτη BMI, την ηλικία και τον δείκτη μάζας λίπους.13 Μελέτη στις ΗΠΑ αποπειράθηκε να προβλέψει ποιοι ασθενείς με βρογχεκτασία ενδέχεται να νοσούν επίσης από NTM-PD.14 Στη συγκεκριμένη μελέτη, τα αποτελέσματα υπέδειξαν ότι σε περισσότερα από δύο αιτήματα καταβολής αποζημίωσης, τα οποία κατατέθηκαν σε ασφαλιστή υγείας στη διάρκεια 12 μηνών και με 30 ημέρες χρονική απόσταση μεταξύ τους, αναφερόταν η διάγνωση πνευμονικής λοίμωξης από NTM σε ασθενείς που είχαν επίσης βρογχεκτασία. Ωστόσο, οι συντάκτες της μελέτης σημείωναν ότι η ευαισθησία πρόβλεψης είναι χαμηλή και, συνεπώς, η πραγματική συχνότητα εμφάνισης μπορεί να έχει υποτιμηθεί σημαντικά. Επιπλέον, μια πρόσφατη μελέτη11 κατέδειξε ότι, παρόλο που η NTM-PD στους ασθενείς με βρογχεκτασία συνδέεται με απεικονιστικές μεταβολές και επιδεινούμενα συμπτώματα, και πάλι η Βαθμολογία Βαρύτητας Βρογχεκτασιών (BSI) μπορεί να παραμείνει αμετάβλητη. Κατά συνέπεια, για τους ασθενείς με βρογχεκτασία, ιδίως εκείνους με απεικονιστικές μεταβολές, συνιστάται ο έλεγχος δείγματος πτυέλων για NTM. Παρομοίως, οι κατευθυντήριες οδηγίες ERS και BTS15,16 υποδεικνύουν ότι θα πρέπει να διερευνάται η πιθανότητα νόσησης από NTM-PD για όλους τους ασθενείς με βρογχεκτασία οι οποίοι λαμβάνουν ήδη μονοθεραπεία με μακρολίδια ή είναι υποψήφιοι λήπτες.
|
Σημαντική επισήμανση: Οι ασθενείς με βρογχεκτασία έχουν αυξημένο κίνδυνο νόσησης από NTM-PD, ο οποίος είναι από 44 έως 187,5 φορές μεγαλύτερος απ’ ό,τι για εκείνους χωρίς βρογχεκτασία 3,4 |
Κυστική ίνωση
Η NTM-PD εμφανίζεται όλο και πιο συχνά σε ασθενείς με κυστική ίνωση, σε παγκόσμιο επίπεδο. Οι λόγοι παραμένουν άγνωστοι, αλλά ίσως να σχετίζονται με τη μεγαλύτερη διάρκεια ζωής των ασθενών, την πρόοδο που έχει σημειωθεί στον έλεγχο των βακτηριακών λοιμώξεων, όπως στην περίπτωση του Pseudomonas aeruginosa, καθώς και τη μεγαλύτερη επαγρύπνηση που οδηγεί σε αυξανόμενες διαγνώσεις NTM-PD7,17. Σύμφωνα με τις τρέχουσες εκτιμήσεις μιας μελέτης, τα NTM προκαλούν λοίμωξη στο 32% των ασθενών με κυστική ίνωση.18 Ο κίνδυνος νόσησης από NTM-PD για τους ασθενείς με κυστική ίνωση είναι υψηλός και ο επιπολασμός της λοίμωξης στον συγκεκριμένο πληθυσμό αυξημένος, μεγαλύτερος κατά 6,6 έως και 13 φορές.6,7
Μια μετα-ανάλυση απέδειξε ότι οι ασθενείς με κυστική ίνωση έχουν σημαντικά μεγαλύτερη πιθανότητα να είναι θετικοί σε καλλιέργειες για NTM αν είναι μεγαλύτερης ηλικίας (p<0,01), έχουν αποικισμό από Aspergillus fumigatus (OR 3,59 p<0,001), Staphylococcus aureus (OR 1,66 p=0,001) ή Stenotrophomonas maltophilia (OR 3,41 p<0,01), ή λαμβάνουν εισπνεόμενα κορτικοστεροειδή (OR 1,98 p<0,01). Καμία άλλη παράμετρος δεν έδειξε σημαντική συσχέτιση19. Λαμβάνοντας υπόψη όλα τα δεδομένα, οι ασθενείς με κυστική ίνωση, καθώς και σοβαρές παθήσεις ή λοιμώξεις, θα πρέπει να παρακολουθούνται στενά για NTM-PD.
|
Σημαντική επισήμανση: Στην περίπτωση της κυστικής ίνωσης, ο κίνδυνος νόσησης από NTM-PD είναι αυξημένος, με τα ποσοστά της NTM-PD στους ασθενείς με κυστική ίνωση να είναι έως και 13 φορές υψηλότερα απ’ ό,τι στον γενικό πληθυσμό (Olivier 2003), ιδίως σε ασθενείς μεγαλύτερης ηλικίας και σε αυτούς με αποικισμό από βακτήρια ή σε αγωγή με εισπνεόμενα κορτικοστεροειδή19 |
ΧΑΠ
Η ΧΑΠ είναι μια συνήθης συννοσηρότητα της NTM-PD και μια μελέτη προγνωστικής μοντελοποίησης 20 υπέδειξε ότι συνιστά έναν από τους υψηλότερους μεμονωμένους προγνωστικούς δείκτες για τη NTM-PD. Στις ΗΠΑ, τα ποσοστά περιστατικών NTM-PD σε ασθενείς με ΧΑΠ αυξάνονται συνεχώς από το 2012 21 και, μάλιστα, υπερδιπλασιάστηκαν στο διάστημα από το 2011 έως το 2015 (σε σύγκριση με αύξησή τους κατά ένα τέταρτο στο διάστημα από το 2001 έως το 2005). Ο κίνδυνος θνητότητας για ασθενείς με ΧΑΠ και λοίμωξη από ΝΤΜ είναι μεγαλύτερος κατά 1,43 φορές απ’ ό,τι για τους ασθενείς με ΧΑΠ που δεν νοσούν από NTM-PD 21, ενώ καναδική μελέτη υπέδειξε ότι το OR για τη ΧΑΠ συνιστά παράγοντα κινδύνου της τάξης του 15,7.4
Μικρότερης εμβέλειας μελέτη κατέδειξε ότι, εφόσον δεν ληφθούν υπόψη ο δείκτης BMI, ο βιαίως εκπνεόμενος όγκος (FEV1) και η χρήση εισπνεόμενων κορτικοστεροειδών, τα ποσοστά της NTM-PD στους ασθενείς με ΧΑΠ είναι υψηλότερα απ’ ό,τι στον γενικό πληθυσμό.22 Παρομοίως, ο λόγος επικινδυνότητας (hazard ratio, HR) για τη NTM-PD στους ασθενείς με προϋπάρχουσα ΧΑΠ ήταν 9,15 μετά από προσαρμογή για το φύλο και την ηλικία και έπεσε μόλις στο 6,01 ακόμα και μετά από πλήρη προσαρμογή για όλους τους παράγοντες.23 Τα δεδομένα αυτά είναι σημαντικά, καθώς η μελέτη του Marras κ.ά. διεξήχθη σε πληθυσμό άνω των έξι εκατ. ατόμων και η συσχέτιση της NTM-PD ως υποκείμενης συννοσηρότητας με τη ΧΑΠ διερευνήθηκε αναλυτικά.
Αν λάβουμε υπόψη τη σταθερή αύξηση των συνολικών ποσοστών της ΧΑΠ, καθώς και τον κίνδυνο νόσησης από NTM-PD για τους ασθενείς με ΧΑΠ, μεταβολές στα συμπτώματα ή απεικονιστικές μεταβολές θα πρέπει να διερευνώνται περαιτέρω με τη λοίμωξη από NTM να είναι ένα από τα πρώτα ενδεχόμενα που θα πρέπει να εξετάζονται.
|
Σημαντική επισήμανση: Όταν παραβλέπονται άλλοι παράγοντες κινδύνου όπως ο δείκτης BMI, η πνευμονική λειτουργία και η χρήση εισπνεόμενων κορτικοστεροειδών, η ΧΑΠ συνεχίζει να συνιστά παράγοντα αυξημένου κινδύνου – έως και 15,7 φορές μεγαλύτερου – για λοίμωξη από NTM3,22,23 |
Άσθμα
Είναι γνωστό ότι οι ασθενείς που λαμβάνουν εισπνεόμενα κορτικοστεροειδή (ICS) έχουν αυξημένο κίνδυνο λοίμωξης από NTM.4,24 Σύμφωνα με μια μελέτη, σε συνδυασμό με το άσθμα per se, ο κίνδυνος για NTM-PD αυξάνεται κατά 7,8 φορές περισσότερο4. Σε μελέτη ασθενών-μαρτύρων από τον ίδιο πληθυσμό διερευνήθηκε συγκεκριμένα η σύνδεση μεταξύ άσθματος και λοίμωξης από NTM και υποδείχτηκε ότι οι ασθενείς με άσθμα είναι μεγαλύτερης ηλικίας, παρουσιάζουν σοβαρό περιορισμό της ροής αέρα και λαμβάνουν εισπνεόμενα κορτικοστεροειδή για μεγαλύτερα χρονικά διαστήματα (>5 έτη) και σε υψηλότερες δόσεις (Hojo 2012). Οι συντάκτες επεσήμαναν ότι η λήψη ICS από τους συγκεκριμένους ασθενείς μπορεί να είναι όντως παράγοντας κινδύνου που συμβάλλει στη λοίμωξη. Λαμβάνοντας υπόψη όλα τα δεδομένα, το άσθμα με τη χαρακτηριστική φλεγμονή και απόφραξη των αεραγωγών είναι λογικό να αποτελεί έναν ανεξάρτητο παράγοντα κινδύνου για τη NTM-PD τον οποίο πιθανώς να ενισχύει ακόμα περισσότερο η χρήση των εισπνεόμενων κορτικοστεροειδών.
|
Σημαντική επισήμανση: Το άσθμα αυξάνει τον κίνδυνο νόσησης από NTM-PD έως και 7,8 φορές περισσότερο4.
|
ς παθή
σεις
Ανοσοκατεσταλμένοι ασθενείς
Είναι γνωστό εδώ και καιρό ότι η χρήση εισπνεόμενων κορτικοστεροειδών αυξάνει τον κίνδυνο για πνευμονία.25 Μελέτη σε ασθενείς άνω των 60 ετών, με άσθμα, ΧΑΠ ή σύνδρομο αλληλεπικάλυψης ΧΑΠ - άσθματος, αξιολόγησε και συνέκρινε τις επιπτώσεις από τη χρήση ή όχι εισπνεόμενων κορτικοστεροειδών σε εκείνους με NTM-PD. Η υφιστάμενη χρήση εισπνεόμενων κορτικοστεροειδών σχετίστηκε με αυξημένο λόγο OR για NTM-PD ίσο με 1,86, με στατιστικώς σημαντική αύξηση στην περίπτωση της φλουτικαζόνης (OR 2,09) έναντι της βουδεσονίδης (OR 1,19), ενώ η σχέση μεταξύ νόσου και λήψης ICS ήταν δοσοεξαρτώμενη.24
Στη ρευματοειδή αρθρίτιδα (RA), η λήψη βιολογικών παραγόντων όπως αυτών που στοχεύουν σε παράγοντες νέκρωσης όγκων (αντι-TNF παράγοντες) σχετίζεται με αυξημένο κίνδυνο νόσησης από NTM-PD 26, ο οποίος προσεγγίζει τα ανώτατα επίπεδα στην περίπτωση της αδαλιμουμάμπης έναντι της ινφλιξιμάβης και ετανερσέπτης: οι ασθενείς που λαμβάνουν τη συγκεκριμένη αγωγή είναι σε 5–10 φορές μεγαλύτερο κίνδυνο απ' ό,τι οι ασθενείς με ρευματοειδή αρθρίτιδα που δεν εκτίθενται σε αγωγή αντι-TNF ή ο γενικός πληθυσμός.26,27 Παρομοίως, σε ασθενείς στη Νότια Κορέα στους οποίους χορηγούταν αγωγή αντι-TNF, αναφέρθηκε υψηλότερη συχνότητα εμφάνισης της NTM-PD με 230 περιστατικά ανά 100.000 ασθενείς,27 ,ενώ στις ΗΠΑ και τη Νότια Κορέα το 70–100% των ασθενών με ρευματοειδή αρθρίτιδα και υποκείμενο πνευμονικό νόσημα είχε NTM-PD, γεγονός που υποδεικνύει συσσώρευση του κινδύνου 27. Ο Prevots κ.ά. εκτιμούν ότι ο αυξημένος κίνδυνος για νόσηση από NTM-PD για τους ασθενείς σε ανοσορυθμιστική ή ανοσοκατασταλτική αγωγή είναι συνολικά από 1,3 έως 2,2 φορές μεγαλύτερος.3
Κι άλλοι βιολογικοί παράγοντες όπως η ριτουξιμάμπη (η οποία χρησιμοποιείται, μεταξύ άλλων, για τον καρκίνο και τη ρευματοειδή αρθρίτιδα), η αμπατασέπτη, η τοσιλιζουμάμπη και η ουστεκινουμάμπη ενέχουν θεωρητικά αυξημένο κίνδυνο νόσησης από NTM-PD, αλλά στην παρούσα περίοδο δεν υπάρχουν διαθέσιμες έρευνες και τα δεδομένα προέρχονται αποκλειστικά από περιορισμένη σειρά περιστατικών.27 Παρομοίως, ασθενείς λήπτες μοσχεύματος που λάμβαναν ανοσοκατασταλτικά φάρμακα, όπως το tacrolimus, διαγνώστηκαν με NTM-PD, αλλά τα δεδομένα προέρχονται και πάλι από περιορισμένη σειρά περιστατικών ή αναφορές μεμονωμένων περιστατικών.28,29
Αν και συμβαίνει πλέον σπάνια, είχε παρατηρηθεί αύξηση των περιστατικών NTM-PD πρωτίστως σε άτομα με HIV/AIDS λόγω της ανοσοκατεσταλμένης κατάστασής τους.27 Με την έλευση της αντιρετροϊκής αγωγής υψηλής δραστικότητας (HAART) υπήρξε κατακόρυφη πτώση των περιστατικών NTM-PD και πλέον η διάγνωση της νόσου μεταξύ των ατόμων που ζουν με τον ιό HIV σπανίζει.
Ασθενείς που λαμβάνουν ανοσοκατασταλτική αγωγή, ιδίως εκείνοι με υποκείμενο πνευμονικό νόσημα, θα πρέπει να ελέγχονται προληπτικά όταν υφίσταται κλινική υποψία. Επίσης, είναι εξαιρετικά σημαντικό συνάδελφοι ρευματολόγοι ή χειρουργοί με εξειδίκευση στις μεταμοσχεύσεις να αντιλαμβάνονται τον θεωρητικό κίνδυνο της ανοσοκατασταλτικής αγωγής σε σχέση με τη NTM-PD, ώστε να μπορούν να παραπέμψουν τον ασθενή κατάλληλα και εγκαίρως.
|
Σημαντική επισήμανση: Σε γενικές γραμμές, στην περίπτωση λήψης ανοσορυθμιστικής ή ανοσοκατασταλτικής αγωγής, ο κίνδυνος για νόσηση από NTM-PD αυξάνεται από 1,5 έως 2,2 φορές περισσότερο.3 |
Παράγοντες κινδύνου του ξενιστή: μορφολογία του ασθενή
Τα χαρακτηριστικά που συνιστούν προδιαθεσιακούς παράγοντες κινδύνου για τη NTM-PD είναι ευρέως γνωστά και περιλαμβάνουν τα ακόλουθα:30
- Βιολογικό φύλο – οι γυναίκες διατρέχουν μεγαλύτερο κίνδυνο
- Παραμορφώσεις του θώρακα όπως, μεταξύ άλλων, σκαφοειδές στέρνο και σκολίωση
- Μεγαλύτερο ύψος από τον μέσο όρο (>165 cm για τις γυναίκες)
- Χαμηλός δείκτης BMI (<20 kg/m2)
- Χαμηλότερο ποσοστό λίπους και κατώτερες μετρήσεις περιφέρειας μηρού σε σύγκριση με τους ασθενείς χωρίς λοίμωξη από NTM.
Τα συγκεκριμένα μορφολογικά χαρακτηριστικά περιγράφηκαν αρχικά ως «σύνδρομο της Λαίδης Γουίντερμιρ», από τον χαρακτήρα του θεατρικού έργου του Όσκαρ Ουάιλντ Η βεντάλια της λαίδης Γουίντερμιρ 31. Ωστόσο, θα πρέπει να σημειωθεί ότι και στους άνδρες, παρόμοια μορφολογικά χαρακτηριστικά σχετίζονται επίσης με αυξημένο κίνδυνο για νόσηση από NTM-PD και ονομάζονται αντιστοίχως «σύνδρομο του λόρδου Γουίντερμιρ».32
Η μορφολογία του ασθενή μπορεί επίσης να επηρεάσει την πορεία της NTM-PD μετά τη διάγνωση, με τους ασθενείς που έχουν χαμηλά ποσοστά κοιλιακού λίπους να διατρέχουν αυξημένο κίνδυνο προοδευτικής εξέλιξης της νόσου.33 Μελέτη ασθενών-μαρτύρων υπέδειξε ότι ασθενείς με ύψος άνω του μέσου όρου και παραμορφώσεις του θώρακα έχουν αυξημένο λόγο συμπληρωματικών πιθανοτήτων (OR) για NTM-PD της τάξης του 1,1 και 5,4 αντίστοιχα, ενώ αποδείχτηκε ότι ένας δείκτης BMI άνω του φυσιολογικού (>26 kg/m2) δρα προστατευτικά έναντι της NTM-PD (OR 0,11)34. Παρομοίως, ο χαμηλός δείκτης BMI σχετίστηκε με αυξημένο κίνδυνο νόσησης από NTM-PD κατά 9,1 φορές περισσότερο.3 Μελέτες οικογενείας υπέδειξαν συρροή περιστατικών NTM-PD μεταξύ συγγενών ασθενών με τα εν λόγω «επικίνδυνα» μορφολογικά χαρακτηριστικά, γεγονός που υποδεικνύει ότι υπάρχει μια υποβόσκουσα γονιδιακή συσχέτιση3,35.
|
Σημαντική επισήμανση: Ορισμένα μορφολογικά χαρακτηριστικά σχετίζονται με αυξημένο κίνδυνο νόσησης από NTM-PD, καθώς το μεγάλο ύψος αυξάνει τον κίνδυνο κατά 1,1 φορές περισσότερο, οι παραμορφώσεις του θώρακα κατά 5,4 φορές περισσότερο και ο χαμηλός δείκτης BMI κατά 9,1 φορές περισσότερο3,34 |
Γενετική προδιάθεση
Ο αυξημένος κίνδυνος νόσησης από NTM-PD σχετίζεται με διάφορες γενετικά κληρονομικές νόσους, στις οποίες περιλαμβάνονται η κυστική ίνωση, όπως επισημάνθηκε παραπάνω, η ανεπάρκεια α1-αντιτρυψίνης (AAT) και η πρωτοπαθής δυσκινησία των κροσσών (ΠΔΚ). Τόσο στην ΑΑΤ όσο και στην ΠΔΚ, η προδιάθεση για τη NTM-PD οφείλεται στον αντίκτυπο της νόσου στον πνεύμονα. Στην AAT, η εμφάνιση της ΧΑΠ είναι ιδιαίτερα συχνό φαινόμενο μετά την ηλικία των 30 ετών36. Στην ΠΔΚ, οι γονιδιακές μεταλλάξεις οδηγούν σε υποβαθμισμένη και ασυντόνιστη λειτουργία των κροσσών στον πνεύμονα, με αποτέλεσμα την πρόκληση βρογχεκτασίας: ο επιπολασμός της NTM-PD στους ασθενείς με ΠΔΚ εκτιμάται ότι ανέρχεται σε ποσοστό περίπου 15%37. Οι ασθενείς με ΠΔΚ θα πρέπει να υποβάλλονται σε προληπτικό έλεγχο για NTM-PD, όπως ακριβώς για την κυστική ίνωση, και συνιστάται τακτική καλλιέργεια πτυέλων κάθε 3–6 μήνες37. Παρομοίως, τόσο στην περίπτωση της κυστικής ίνωσης όσο και της ΠΔΚ, η εκπαίδευση των ασθενών πάνω στην αποτελεσματική κάθαρση των αεραγωγών έχει ζωτική σημασία, καθώς ο καθαρισμός του πνεύμονα προλαμβάνει τη λοίμωξη και, αν ο ασθενής έχει διαγνωστεί με NTM-PD, την πρόκληση βλάβης στον πνεύμονα.
Τα σύνδρομα που συνιστούν πρωτοπαθείς ανοσοανεπάρκειες, όπως η κληρονομική ευαισθησία σε μυκοβακτηριακές λοιμώξεις (MSMD), μια σπάνια πάθηση, αποτελούν παράγοντα κινδύνου νόσησης από NTM-PD. Η MSMD σχετίζεται με διαταραχές του υποδοχέα ιντερλευκίνης και μεταλλάξεις γονιδίων που συμμετέχουν στη φλεγμονώδη απόκριση 38. Παρομοίως, ποικίλες γενετικές διαταραχές στη φλεγμονώδη οδό όπως η ιντερλευκίνη (IL-12) της ιντερφερόνης-γ (IFNγ) και οι υποδοχείς IFN/IL, καθώς και πρωτεΐνες μακροφάγων όπως το γονίδιο NRAMP1 (πρωτεΐνη μακροφάγων που σχετίζεται με τη φυσική αντίσταση 1), είναι γνωστοί παράγοντες κινδύνου νόσησης από NTM-PD39, υποδεικνύοντας πιθανότατα μια συσχέτιση φλεγμονώδους αντίδρασης και νόσου.
Πρωθύστερη ή υφιστάμενη φυματίωση
Σε πρόσφατη μελέτη40 στην Κίνα, μια αξιολόγηση των περιστατικών NTM-PD σε ασθενείς με φυματίωση υπέδειξε ότι ένας στους δεκαπέντε έχει επίσης λοίμωξη από NTM. Τα συνηθέστερα λοιμογόνα είδη ήταν το M. intracellulare και το M. abscessus. Ένα παρεμφερές περιστατικό συλλοίμωξης παρουσιάστηκε μερικά χρόνια πριν, με τους συντάκτες της μελέτης να υποδεικνύουν ότι η λοίμωξη από το μυκοβακτηρίδιο της φυματίωσης προκαλείται στην πλειονότητα των ασθενών στους πρώτους έξι μήνες από τη διάγνωση της NTM-PD και ότι είναι κατά πολύ συχνότερη σε άτομα με ιστορικό πρωθύστερης NTM-PD41. Αντιστρόφως, όπως υποδείχτηκε, στο ΗΒ η πρωθύστερη φυματίωση ως παράγοντας κινδύνου για τη NTM-PD επιφέρει λόγο OR της τάξης του 69,0, ενώ στη Δανία υπήρξε υπερδιπλασιασμός του κινδύνου με OR της τάξης του 178,34,42.
Παρόλο που η φυματίωση φαίνεται να υποχωρεί στην Ευρώπη 43, συνεχίζει να είναι ενδημική σε ορισμένες χώρες και η άνοδος των περιστατικών φυματίωσης ανθεκτικής στη ριφαμπικίνη και σε πολυφαρμακευτικά σχήματα θεραπείας σημαίνει ότι η νόσος παραμένει μια σημαντική παράμετρος που θα πρέπει να λαμβάνεται υπόψη. Όταν παρουσιάζεται ένας ασθενής με συμπτώματα NTM-PD και γεννάται η υποψία λοίμωξης, οι ασθενείς θα πρέπει να υποβάλλονται πάντα σε προληπτικό έλεγχο για πρωθύστερη φυματίωση.
Γαστροοισοφαγική παλινδρόμηση (ΓΟΠ)
Στους υπόλοιπους προδιαθεσιακούς παράγοντες κινδύνου περιλαμβάνεται η ΓΟΠ44 με αυξημένο κίνδυνο που εκτιμάται να είναι κατά 1,5–5,3 φορές μεγαλύτερος.3 Οι ασθενείς με ΓΟΠ είναι πιθανότερο να έχουν θετική οξεάντοχη χρώση των βακτηρίων ΝΤΜ και να νοσούν από NTM-PD σε σύγκριση με τους ασθενείς χωρίς ΓΟΠ. Παρομοίως, στους ασθενείς με πνευμονική νόσο από MAC (MAC-PD), εμφανίζεται ένα υψηλό ποσοστό περιστατικών με τη ΓΟΠ ως συννοσηρότητα 44,45. H ΓΟΠ εμφανίζεται συχνότερα σε ασθενείς με NTM-PD με παρουσία οζιδίων σε ποσοστό περίπου 26%45, ακόμα κι αν γίνει προσαρμογή για άλλους παράγοντες όπως η ηλικία, το φύλο, ο δείκτης BMI και οι εξετάσεις πνευμονικής λειτουργίας. Ασθενείς με ΓΟΠ και NTM-PD που λαμβάνουν οξεοκατασταλτικά φάρμακα είναι πιθανότερο να έχουν εμφάνιση πύκνωσης και οζίδια με μέγεθος άνω των 5 mm.44 Η ΓΟΠ πιθανώς να συνιστά παράγοντα κινδύνου λόγω της αναρρόφησης βακτηρίων της χολής από τον πνεύμονα και θεωρείται ότι η καταστολή οξέος υποστηρίζει τον πολλαπλασιασμό και την επιβίωση των βακτηρίων στη χολή.44
Περιβαλλοντική έκθεση
Τα NTM είναι πανταχού παρόντα στο περιβάλλον, απαντώνται τόσο στο έδαφος όσο και στο νερό 46–48 και έχει εκφραστεί η άποψη ότι το περιβάλλον αποτελεί τη «δεξαμενή» των συγκεκριμένων βακτηρίων που προκαλούν λοίμωξη στον ανθρώπινο οργανισμό. Ο αυξανόμενος επιπολασμός της NTM-PD έχει σχετιστεί με τα αυξημένα επίπεδα υδρατμών στην ατμόσφαιρα 49 και η παρουσία NTM στο νερό οικιακής χρήσης αναφέρεται συχνότερα για τους ασθενείς με NTM-PD απ’ ό,τι για τους μη νοσούντες50, αυξάνοντας τον κίνδυνο νόσησης από NTM-PD έως και 5,9 φορές περισσότερο3. Τα NTM είναι επίσης παρόντα και σε ένα πλήθος άλλα κλινικά περιστατικά που σχετίζονται με το εξωτερικό και το οικιακό περιβάλλον, αλλά ο ρόλος τους στη λοίμωξη παραμένει ασαφής2. Με δεδομένη τη διεισδυτική φύση των ΝΤΜ, ο περιορισμός της λοίμωξης είναι δύσκολος, αλλά απλά μέτρα προφύλαξης όπως η τακτική αντικατάσταση των φίλτρων νερού, η αποφυγή λουτρών υδρομασάζ, ο τακτικός καθαρισμός της κεφαλής ντους και η χρήση γαντιών κατά τις κηπουρικές εργασίες μπορούν να βοηθήσουν τους ασθενείς σε κίνδυνο.
Συνοπτικά
Από τις όλο και περισσότερες έρευνες σε εξέλιξη, καθίσταται σαφές ότι υπάρχει μια σειρά δυνητικών παραγόντων κινδύνου που μπορούν να εγείρουν την κλινική υποψία για NTM-PD. Ωστόσο, είναι γνωστό ότι ούτε κάθε ψηλή, λεπτή γυναίκα ούτε κάθε ασθενής με ΓΟΠ, αν και με αυξημένο παράγοντα κινδύνου για NTM-PD, θα νοσήσει και, συνεπώς, η περαιτέρω διερεύνηση με βάση την κλινική υποψία έχει θεμελιώδη σημασία. Στο πλαίσιο αυτό, μεταξύ άλλων, θα πρέπει να λαμβάνονται υπόψη μεταβολές ή επιδείνωση στην κλινική κατάσταση του ασθενή με υψηλό κίνδυνο για NTM-PD και να μελετάται το ιατρικό και κοινωνικό ιστορικό, προκειμένου να γίνονται αντιληπτοί οι ιατρογενείς κίνδυνοι στη ζωή του ασθενή. Στη συνέχεια, η διεξαγωγή εξετάσεων και η παρακολούθηση του ασθενή, όταν υφίσταται υποψία για νόσηση από NTM-PD, έχει επιτακτική σημασία.
|
Σημαντική επισήμανση: Διεξάγετε εξετάσεις και παρακολουθείτε τους ασθενείς με δυνητικά κλινικά συμπτώματα ή επιδεινούμενα κλινικά συμπτώματα οι οποίοι ταιριάζουν με το προφίλ της νόσου NTM-PD: · Ψηλοί, λεπτοί άντρες ή γυναίκες · Ασθενείς με υποκείμενες πνευμονικές παθήσεις: άσθμα, βρογχεκτασία και ΧΑΠ · Ασθενείς με γενετικά κληρονομικές παθήσεις: κυστική ίνωση, ΑΑΤ, ΠΔΚ · Ασθενείς με ΓΟΠ ή περιβαλλοντική έκθεση |
Τι πρέπει να κάνετε; Διερευνήστε την πιθανή ύπαρξη ΝΤΜ για τους ασθενείς με παράγοντες κινδύνου, ώστε να διαγνώσετε εγκαίρως τη λοίμωξη, και ακολουθήστε τις τρέχουσες κατευθυντήριες οδηγίες (Daley 2020) για την έναρξη της θεραπείας, προκειμένου να προληφθεί η προοδευτική εξέλιξη της νόσου και να επιτευχθεί η αρνητικοποίηση της καλλιέργειας. Σκέψου τα NTM! Κάνε εξετάσεις για NTM!
Βιβλιογραφία:
1. Daley CL, et al. Eur Respir J 2020;56:2000535.
2. Cowman S, et al. Eur Respir J 2019;54:1900250.
3. Prevots DR, et al. Clin Chest Med 2015;36:13–34.
4. Andrejak C, et al. Thorax 2013;68:256–62.
5. Axson EL, et al. Eur J Clin Microbiol Infect Dis 2019;38:117–24.
6. Olivier KN et al. Am J Respir Crit Care Med 2003;167:828-34
7. Roux A-L, et al. J Clin Microbiol 2009;47:4124–28.
8. Hojo M, et al. Respirology 2012;17:185–90.
9. Shteinberg M, et al. Eur Respir J 2018; 51:1702469.
10. Ringshausen FC, et al. Emerg Infect Dis 2016;22:1102–05.
11. Chu H, et al. Arch Med Sci 2014;10:661–68.
12. Lim SY, et al. Medicine (Baltimore) 2021;100:e25193.
13. Ku JH, Diagn Microbiol Infect Dis 2020;96:114916.
14. Kwak N, et al. BMC Pulm Med 2020;20:293.
15. Smith D, et al. Thorax 2020;0:1–35.
16. Polverino E, et al. Eur Resp J 2017 ;50 :1700629.
17. Salsgiver EL, et al. Chest 2016;149:390–400.
18. Floto RA, et al. Thorax 2016;71:88–90.
19. Reynaud Q, et al. Pediatr Pulm 2020;55:2653–61.
20. Ringshausen FC, et al. Int J Infect Dis 2021;104:398–406.
21. Pyarali FF, et al. Front Med 2018;5:311.
22. Okamuri S, et al. Eur Respir J 2015;46:PA569.
23. Marras TK, et al. Eur Respir J 2016;48:928–31.
24. Brode SK, et al. Eur Respir J 2017;50:1700037.
25. Suissa S, et al. Thorax 2013;68:1029–36.
26. Winthrop KL, et al. Ann Rheum Dis 2013;72:37–42.
27. Henkle E, et al. Clin Chest Med 2015;36:91–99.
28. Suzuki H, et al. Transplant Proc 2018;50:2764–67.
29. Imoto W, et al. BMC Infect Dis 2020;20:431.
30. Kim RD, et al. Am J Respir Crit Care Med 2008;178:1066–74.
31. Reich JM, et al. Chest 1992;101:1605–09.
32. Ku JH, et al. Emerg Infect Dis 2021;27:982–85.
33. Kim SJ, et al. BMC Pulm Med 2017;17:5.
34. Dirac MA, et al. Am J Respir Crit Care Med 2012;186:684–91.
35. Colombo RE, et al. Chest 2010;137:629–34.
36. Stoller JK, et al. 2006 Oct 27 [Updated 2020 May 21]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2021. https://www.ncbi.nlm.nih.gov/books/
37. Daniels MLA, et al. Exp Opin Orphan Drugs 2015;3:31–44.
38. Ratnatunga CN, et al. Front Immunol 2020;11:303.
39. Baldwin SL, et al. PLOS Neglected Trop Dis 2019;13:e0007083.
40. Tan Y, et al. J Infect 2021;S0163-4453(21)00261-9.
41. Hsing S-C, et al. Int J Tuberc Lung Dis 2013;17:928–33.
42. European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2019 Accessed July 2021
42. Thomson
43. Koh W-J, et al. Chest 2007;131:1825–30.
43. Lee E-S, et al. J Microbiol Biotechnol 2008;18:1207–15.
44. Falkinham JO. Clin chest Med 2015;36:35–41.
45. Falkinham JO. J Appl Microbiol 2009;107:356–67.
46. Prevots DR, et al. Annals Am Thorac Soc 2014;11:1032–38.
47. Nishiuchi Y, et al. Clin Infect Dis 2007;45:347–51.
Impact of non-tuberculous mycobacteria (NTM) on at-risk patients
Non-tuberculous mycobacteria (NTM) can cause serious pulmonary disease in at-risk patients, which can have a significant impact on health-related quality of life, morbidity and mortality, and increase disease progression in patients with structural lung diseases. Understanding who is at risk can facilitate earlier diagnosis and treatment, which is crucial in preventing disease progression and lung function decline.
Non-tuberculous mycobacteria (NTM) are opportunistic infections that can cause infection at a wide range of body sites in patients who have underlying disease or are immunosuppressed.1 Inhalation of some NTM species in vulnerable people can cause non-tuberculous mycobacterial pulmonary disease (NTM-PD)2. NTM-PD can be caused by a variety of mycobacterial species, the most common of which is the Mycobacterium avium complex (MAC), which comprises two main species M. avium and M. intracellulare. In one study of 62 centres in 30 countries of 18,418 isolates MAC-PD accounted for 47% of incidences of NTM-PD.3
How does NTM-PD have an impact on quality of life?
NTM-PD can be a significant burden on patients. Patients with NTM-PD, including MAC-PD, may have reduced lung function, increased morbidity and mortality, and reduced health-related quality of life (HRQoL) compared with the general population.3,4–11
All-cause mortality in patients with NTM-PD can be up to four times higher than the general population, independent of other factors.10–12 For MAC-PD, studies showed a pooled estimate of five-year all-cause 5-year mortality of 27%.2 NTM-PD can also cause a significant reduction in patients’ lung function.7–9 Patients with NTM-PD have been shown to experience a more substantial reduction in forced expiratory volume in 1 sec (FEV1) compared with those without NTM-PD.8 In one study where patients with mild disease were considered not to require treatment, chronic NTM infection caused a substantial decline in lung function over time.7 NTM-PD is associated with a lower HRQoL compared with the general population, with these patients demonstrating higher scores using the St. George’s Respiratory Questionnaire (SGRQ) and lower scores using the Medical Outcomes Study 36-item Short Form Survey (SF-36).4,13 In one study, SGRQ scores in patients with NTM-PD were over 25 points worse compared with normal values.13 Another study showed that patients eventually requiring treatment for their NTM-PD had worsening SGRQ scores, suggesting an association between disease progression and lower HRQoL.4
Who is most at risk of NTM-PD?
Understanding who is at increased risk of NTM-PD can help in early recognition and diagnosis of disease. High-risk groups include tall, elderly women with a low body mass index (BMI) and abnormalities of the skeleton for example, conditions such as abnormal spinal curvatures (scoliosis, kyphosis) and structural abnormality of the chest where the sternum is pressed inward (pectus excavatum) – so-called Lady Windermere syndrome – patients with underlying lung conditions such as bronchiectasis and chronic obstructive pulmonary disease (COPD), and immunocompromised and immunosuppressed patients; exposure to NTM species is much more likely to cause disease in these groups.6,14–16
Patients with low BMI
There is an association between NTM-PD and marfanoid characteristics of elderly female patients who are taller than average with low body weight, as well as those with thoracic skeletal abnormalities; low body weight alone increases risk of NTM-PD by three-fold and thoracic abnormalities five-fold.17,18 In patients with NTM-PD, low BMI (<18.5 kg/m2) has been associated with the presence of multiple NTM isolates as well as a lower chance of treatment success.4,5 In addition, patients with lower BMI are more likely to fail treatment for their NTM-PD.4
Transplant recipients
NTM can also cause disease in immunosuppressed patients who are recipients of organ or stem cell transplants.6 Rates of NTM infections in lung transplant recipients are particularly high, and have been shown to increase post-transplant mortality, with an estimated 5-year mortality of 50%.6
Patients with structural lung diseases
NTM can cause pulmonary disease in patients with pre-existing underlying lung conditions, such as those with bronchiectasis (44.0–187.5 increased risk) and COPD (2.0–10.0 increased risk).15,16 Infection and inflammation caused by NTM in such patients can lead to deterioration of pulmonary function, causing faster disease progression compared with patients without NTM-PD.5 In one study, the presence of multiple NTM isolates in patients with COPD has been associated with a greater decline in FEV1 as well as an increase in exacerbations requiring hospitalisation compared with the absence of isolates.5
Act now for your at-risk patients
Early diagnosis and treatment of NTM-PD is crucial in preventing disease progression and declining lung function.19
NTM-PD is often misdiagnosed or diagnosed late, as symptoms of NTM-PD are similar to those of coexisting lung disease and may be present for more than 10 years before diagnosis; increasing testing for your at-risk patients may lead to a higher chance of spotting NTM-PD early.20,21 In a survey of 280 hospital-based physicians, the majority perceived NTM-PD as a significant factor for worsening respiratory function, increasing morbidity and hospitalisation in patients with bronchiectasis, although fewer perceived NTM-PD as having a significant impact on mortality.22 However, the mortality rate for NTM-PD and in particular MAC-PD is high (up to 27%),2 so recognising the risk of NTM-PD and testing your at-risk patients is important, as missing a diagnosis can lead to worse long-term outcomes for your patients, including increased risk of death.
At-risk patients with underlying lung conditions such as COPD or bronchiectasis may receive long-term macrolide monotherapy to prevent exacerbations of underlying disease.23 One study indicated that 42% of patients with bronchiectasis received macrolide monotherapy, however, macrolide monotherapy is not recommended in patients with NTM-PD because of the increased chance of macrolide resistance.22
Guidelines for managing bronchiectasis outline that any patient being considered for macrolide therapy for exacerbations should be screened for underlying NTM to rule out infection and protect antimicrobial susceptibility for NTM therapy.23
Currently, 68% of healthcare professionals do not test their patients for NTM-PD prior to initiating macrolide treatment, despite 87% perceiving these patients to be at particular risk of NTM infection.22 In fact, macrolide monotherapy is one of the major predispositions for macrolide-resistant MAC,24 so testing your at-risk patients for NTM-PD prior to initiating macrolide monotherapy is essential to reduce the emergence of macrolide-resistant NTM-PD and to increase the chance of cure. In patients with macrolide-resistant NTM-PD, treatment outcomes are poor, with an estimated all-cause 5-year mortality rate of 47%.25
Βιβλιογραφία:
- Centers for Disease Control and Prevention. Nontuberculous mycobacteria (NTM) infections. https://www.cdc.gov/hai/organisms/nontuberculous-mycobacteria.html [Accessed March 2021]
- Diel R, et al. BMC Infect Dis 2018;18:206.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Kwak N, et al. BMC Pulm Med 2020;20:126.
- Huang CT, et al. Int J Tuberc Lung Dis 2012;16:539–45.
- Friedman DZP, et al. Transpl Infec Dis 2020;22:e13229.
- Park HY, et al. Chest 2016;150:1222–32.
- Kobayashi T, et al. J Clin Tuberc Other Mycobact Dis 2018;11:17–21.
- Lee MR, et al. PLoS One 2013;8(3):e58214.
- Marras TK, et al. Respir Med 2018;145:80–8.
- Fleshner M, et al. Int J Tuberc Lung Dis 2016;20:582–7.
- Diel R, et al. Eur Resp J 2017;49:1602109.
- Mehta M, et al. Resp Med 2011;105:1718–25.
- Aksamit TR, et al. Chest 2017;151:982–92.
- Andrejak C, et al. Thorax 2013;68:256–62.
- Prevots DR, et al. Clin Chest Med 2015;36:13–34.
- Dirac MA, et al. Am J Respir Crit Care Med 2012;186:684–91.
- Axson EL, et al. Eur J Clin Microbiol Infect Dis 2019;38:117–24.
- Park TY, et al. PLoS One 2017;12:e0185774.
- Kotilainen H, et al. Eur J Clin Microbiol Infect Dis 2015;34:1909–18.
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Wagner D, et al. BMJ Open Respir Res 2020;7:e000498.
- Smith D, et al. BMJ Open Resp Res 2020;7:e000489.
- Griffith DE, et al. Curr Opin Infect Dis 2012;25:218–27.
- Moon SM, et al. Antimicrob Agents Chemother 2016;60:6758–65.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Ongoing or recently completed clinical trials in NTM-PD
(Information correct as of May 2021)
NTM-PD has been a ‘Cinderella’ disease for many years, but is now subject to an increasing number of clinical studies that explore treatment of NTM-PD, epidemiology and NTM-PD patient registries.
This section provides an overview of ongoing clinical trials for NTM-PD that are listed on clinical trial registers in the EU (EudraCT) and the USA (clinicaltrials.gov). Information will be supplemented and updated over time.
Clinicaltrials.gov: https://www.clinicaltrials.gov
EudraCT: https://www.clinicaltrialsregister.eu/ctr-search/search/
All information contained in this section is correct as of May 2021
|
Clinical trial notifier |
Study overview |
Endpoints |
Status |
|
NCT04294043 https://clinicaltrials.gov/ct2/show/
|
Evaluating the efficacy of intravenous (IV) gallium nitrate in patients with cystic fibrosis (CF) who are colonised with NTM: the ABATE study |
The ABATE study is a Phase IIb, multicentre study from the Cystic Fibrosis Foundation in the USA to evaluate the efficacy of IV gallium nitrate in patients with CF whose lungs are colonised with NTM.
The primary endpoint is the proportion of patients who discontinue because they are experiencing one or more adverse events of special interest from baseline to day 57.
Secondary endpoints include laboratory measurements (day 0–57) and clearance of NTM from the lungs as measured by two sequential NTM cultures (day 0–111).
The ABATE study is due to start in 2021 and complete in early 2023.
|
Recruiting |
|
NCT02779478 https://clinicaltrials.gov/ct2/show/ |
Evaluation of the lung microbiome in NTM bronchiectasis |
This study, based in the USA, is designed to explore alterations in the microbiome of patients with NTM-PD.
This study will use induced sputum and upper airway samples (via bronchoscopy) to evaluate microbiome species using quantitative polymerase chain reaction (qPCR) as the primary endpoint. Two hundred patients are planned for enrolment and will include patients with active NTM-PD and culture-negative patients as a control group.
Secondary endpoints will include: · the Eating Assessment Tool (EAT) to evaluate the presence of laryngopharyngeal reflux and dysphagia/aspiration in both groups. · laryngoscopy to calculate the Reflex Finding Score (RFS) across patients. · the Reflux Symptom Index (RSI) to evaluate the prevalence of laryngopharyngeal reflux and dysphagia/aspiration.
The study is due to complete in mid-2024. |
Recruiting |
|
NCT03339063 https://clinicaltrials.gov/ct2/show/ |
Italian REgistry of Pulmonary Non-tuberculous mycobactEria (IRENE) |
IRENE is one of several registries being established for NTM-PD. IRENE is an observational, multicentre, prospective study enrolling patients with NTM-PD. Currently, IRENE includes 35 centres and provides an insight into real-world clinical practice and management of NTM-PD.
IRENE will also contain a biobank linked to the clinical registry that will consist of blood, serum, plasma and respiratory samples.
|
Ongoing – no end date |
|
NCT04334070 https://clinicaltrials.gov/ct2/show/ |
Lamprene Expanded Access Programme |
Lamprene (clofazimine) was previously used as treatment for leprosy and is not licensed for use in NTM-PD. Clofazimine 50 mg twice daily is now being studied through an expanded access programme in patients with drug-resistant NTM or in those where side-effects mean continuing with guideline-based therapy is not possible.
All patients in the programme will have NTM-PD or disseminated NTM infection and have failed or are intolerant to previous therapies or have macrolide resistance at baseline.
No endpoints for study outcomes are provided. Similarly, no timeframes for study completion are available.
|
Open to patients |
|
NCT04024423 https://clinicaltrials.gov/ct2/show/ |
Transmission of NTM in CF: the HALTNTM study |
Sources of infection for NTM and transmission among patients with CF are poorly understood, but person-to-person transmission within healthcare settings is suspected.
The Healthcare-Associated Links in Transmission of Nontuberculous Mycobacteria in Cystic Fibrosis (HALTNTM) study in the USA is an observational study to identify the potential mechanism of healthcare-associated transmission and the source of infection. It takes a systematic approach to examine infection clusters of NTM-PD in patients with CF within the same CF centre. Patients will be identified using whole genome sequencing to identify NTM strains within single CF centres. Species for study inclusion include Mycobacterium avium, M. intracellulare and M. abscessus.
The primary endpoints are to identify the number of patients with CF and NTM who are part of an infection cluster within a single CF centre and to compare patient’s NTM isolates with environmental isolates.
The study started in 2019 and is due to complete in mid-2022.
|
Recruiting |
|
NCT04579211 https://clinicaltrials.gov/ct2/show/ |
Urinalysis as a screening tool for NTM-PD in patients with CF |
This study is a single-centre, prospective observational study at National Jewish Health in the USA to explore if urinalysis can provide a non-invasive method to screen for NTM in patients with CF.
Patients will provide three urine samples that are timed to be collected alongside routine sputum samples. Urine will then be screened for NTM isolates to identify those individuals at low risk of being culture positive for NTM.
The study started in December 2020 and is due to complete in late 2023.
|
Recruiting |
|
NCT03672630 https://clinicaltrials.gov/ct2/show/ |
Comparison of a two- and a three-drug regimen for treating Mycobacterium avium complex pulmonary disease (MAC-PD) |
This US study explores the efficacy of a two-drug regimen for patients with active MAC-PD compared with the guideline-recommended three-drug regimen to explore if three drugs are necessary and if the use of two drugs could reduce side-effects.
Patients will receive either intermittent (dosed three times per week) azithromycin 500 mg plus ethambutol 25 mg/kg or azithromycin 500 mg plus ethambutol 25 mg/kg plus rifampicin/rifampin 600 mg. The co-primary endpoints of the study will be culture conversion by 12-months post-randomisation and the proportion of patients completing 12 months of therapy.
Secondary endpoints will focus on quality of life, symptoms, fatigue and the appearance of adverse events, as well as the evolution of macrolide resistance.
The study started in early 2019 and is due to complete in early 2023.
|
Recruiting |
|
NCT04163601 https://clinicaltrials.gov/ct2/show/ |
Efficacy of amikacin liposomal inhalation suspension (ALIS) in M. abscessus |
This study, based in France, explores the efficacy of ALIS provided on a compassionate-use basis to patients with M. abscessus.
The primary outcome for this study is culture conversion, microbiological and clinical cure at the end of treatment.
Outcome definitions are defined by NTM-NET [https://pubmed.ncbi.nlm.nih.gov/29567726/].
The study is due to complete in early 2024.
|
Recruiting |
|
NCT04677543 https://clinicaltrials.gov/ct2/show/
EudraCT: 2020-002545-42
|
Validating patient-reported outcomes (PROs) in patients with NTM-PD: the ARISE study |
The aim of the Phase III ARISE study is to validate the Quality of Life – Bronchiectasis (QoL-B) and the Patient-Reported Outcome Measurement Information system – Fatigue-Short Form (PROMIS F-SF 7a) in patients with newly diagnosed NTM-PD caused by MAC.
Throughout the study other tools will be completed to anchor the results of QoL-B and PROMIS F-SF 7a and include the: · EXAcerbations of Chronic pulmonary disease tool (EXACT) · EXACT respiratory symptoms (EXACT-RS) · St George’s Respiratory Questionnaire (SGRQ) · Functional Assessment of Chronic Illness Therapy – Fatigue Scale (FACIT-Fatigue) · Patient Global Impression of Severity – Respiratory (PGIS-Respiratory) · Patient Global Impression of Severity – Fatigue (PGIS-Fatigue).
The study started in late 2020 and will complete in early 2022.
|
Recruiting |
|
NCT04677569 https://clinicaltrials.gov/ct2/show/
EudraCT: 2020-003079-16 |
Evaluating ALIS in patients with newly diagnosed MAC-PD: the ENCORE study |
The objective of this Phase III study is to evaluate the efficacy of a two-drug regimen against a three-drug regimen for the treatment of newly diagnosed MAC-PD.
Patients in the control group will receive azithromycin 250 mg plus ethambutol 15 mg/kg once daily and, for the purposes of blinding, inhaled empty liposomes. The active treatment group will receive a three-drug regimen of azithromycin 250 mg plus ethambutol 15 mg/kg plus ALIS 590 mg. The dosing regimen in both groups will be daily.
The primary outcome for the study is the change from baseline in respiratory scores at month 13.
Secondary endpoints include: · percentage of patients achieving culture conversion at months 6 and 12 · percentage of patients with a durable culture conversion at month 15 · time to culture conversion · time to first negative culture · change from baseline in fatigue · percentage of patients with emergent resistance to amikacin (≥128 μg/mL) · reinfection with MAC and infection with a new MAC species or the same species but genetically different from the original infection.
The study is due to start in 2021 and complete in 2023.
|
Recruiting |
|
NCT04616924 https://clinicaltrials.gov/ct2/show/ |
Evaluation of RHB-2014 (clarithromycin, rifabutin and clofazimine) in treating MAC-PD: the CleaR-MAC study |
This Phase III study will evaluate the efficacy and safety of RHB-204 in adults with MAC-PD. RHB-204 is a fixed-dose combination therapy containing clarithromycin 158.3 mg, rifabutin 40 mg and clofazimine 13.3 mg.
The study will be a two-part multicentre, randomised, double-blind, placebo-controlled, parallel group study in adults with nodular bronchiectasis and confirmed MAC-PD. Patients in the study will receive either RHB-204 or matching placebo that contains riboflavin to maintain blinding.
The primary endpoint will be sputum-culture conversion by month 6 (defined as at least three previous consecutive negative monthly sputum cultures by month 6). Patients will remain in the study in receipt of medication until month 16, and patient-reported outcomes and durability of culture conversion will be assessed at month 6 and month 19, 3 months after completion of therapy.
Secondary endpoints include changes from baseline in quality of life, reduction in fatigue, time to culture conversion, durability of sputum-culture conversion.
It is estimated CleaR-MAC will recruit 125 patients.
The study started at the end of 2020 and is anticipated to complete in mid-2023. |
Recruiting |
An overview of the rationale and approach to diagnosis of Mycobacterium avium complex pulmonary disease (MAC-PD)
Mycobacterium avium complex pulmonary disease (MAC-PD) is difficult to diagnose with symptoms similar to underlying lung conditions.1 Correct, early diagnosis and treatment are paramount to prevent disease progression.1–4 The 2020 international guidelines recommend clinical, radiographical and microbiological diagnostic criteria for non-tuberculous mycobacterial pulmonary disease (NTM-PD) to facilitate timely and appropriate treatment.5
NTM-PD: an overlooked disease caused by ubiquitous mycobacteria
Non-tuberculous mycobacteria pulmonary disease (NTM-PD) is a chronic and potentially debilitating disease.6–10 Mycobacteria are ubiquitous in the environment11–13 and comprise almost 200 species and subspecies that can cause opportunistic infections in both pulmonary and extrapulmonary sites.5 NTM infections can be difficult to diagnose and treat and are particularly prevalent in those with lung damage or disease, cancer and immunodeficiencies.14
A series of 12 molecularly related Mycobacterium species have been identified that together comprise the Mycobacterium avium complex (MAC).15 Within MAC, the two most clinically relevant species are M. avium and M. intracellulare.16,17 MAC is the most common cause of NTM.18
Figure 1. Worldwide distribution of respiratory NTM isolates.
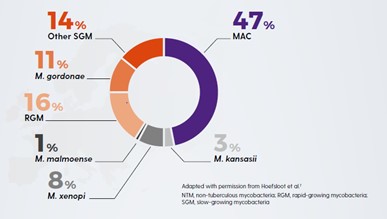
(Adapted from Hoefsloot, 2013)18
Increasing prevalence of NTM-PD
The incidence and prevalence of NTM-PD is increasing in many parts of the world.1,5,19,20 This may reflect increased awareness of the importance of NTM,1 the incidence of risk factors such as chronic obstructive pulmonary disease (COPD) and bronchiectasis,21,22 the use of immunosuppressive treatments,23 testing for NTM-PD and the effectiveness of diagnostic tools.1,17,24 NTM should be ruled out in at-risk patients to identify infection early and begin treatment in appropriate patients before there is disease progression.
Early diagnosis of NTM-PD is paramount to prevent disease progression.
NTM-PD is associated with increased mortality and morbidity – increasing the risk of pulmonary exacerbations9, lung cancer25 and other lung infections26 (e.g. tuberculosis [TB], aspergillosis) and atrial fibrillation.27 NTM-PD is difficult to diagnose as the symptoms of NTM-PD – cough, fatigue, haemoptysis, weight loss – are similar to underlying lung conditions.1 Many patients with NTM-PD may experience symptoms for >10 years before diagnosis.28 Correct, early diagnosis and treatment are paramount to prevent disease progression.1–4 One study has shown that without treatment, 97.5% (n=39/40) of MAC-PD patients will have disease progression within 6 years.17 Another study showed that for those with M. kansasii progression can be very rapid in untreated patients within 1 year (progression in up to 63% of patients) and a median survival of 71 days.29 Late diagnosis, misdiagnosis or inappropriate management of NTM-PD is likely to increase the risk of deterioration in lung health and health-related quality of life.
NTM-PD – not all species are equal
Not all NTM species will cause disease, and when they do, they may not need to be treated.1,30 Likewise the geographical distribution of species differs, driving local epidemiology. Knowledge of the local situation and species virulence is essential for daily clinical practice. MAC is most frequently associated with NTM-PD across all continents with M. avium causing about 63% of infections meeting ATS/IDSA criteria for treatment and M. intracellulare about 88%. Species such as M. gordonae are rarely clinically relevant.30
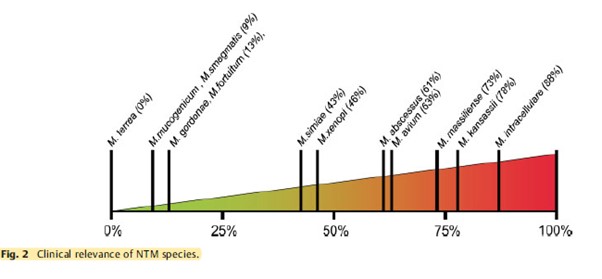
Who is at risk of NTM-PD?
For many at-risk patients NTM-PD symptoms are similar to symptoms of coexisting lung disease.1 These include chronic cough, fatigue, weight loss and low-grade fever.
Patients most at risk of developing NTM-PD include those with:
- Lung diseases e.g. bronchiectasis, COPD, asthma, prior TB.31–34
- Diseases/disorders causing structural lung damage e.g. cystic fibrosis, rheumatoid arthritis, genetic mutation.35–38
- Thoracic skeletal abnormalities – scoliosis, kyphosis, pectus excavatum:39,40 five times more likely to have NTM.34
- Diseases/disorders reducing cell-mediated immunity e.g. HIV AIDS, cancer, genetic mutation.37,38,41
- Therapies resulting in immunodeficiency e.g. organ transplant, anti-tumour necrosis factor (TNF) therapy, corticosteroids, immunosuppressants.34–36,41–44
- Marfanoid body habitus/Lady Windermere syndrome39,40,45 – tall, slender elderly patients with a below normal body mass index (BMI) (<18 Kg/m2) are three times more like to have NTM.34
|
Factors increasing susceptibility to NTM-PD |
Risk* |
|
Bronchiectasis32,47 |
44.0–187.5 |
|
Low BMI47 |
9.1 |
|
Cystic fibrosis65,66 |
6.6–13.0 |
|
COPD47 |
2.0–10.0 |
|
Thoracic skeletal abnormalities47 |
5.4 |
|
Asthma67 |
2.0 |
|
Steroid use47 |
1.6–8.0 |
|
GORD47 |
1.5–5.3 |
|
Immunomodulatory/immunosuppressant therapies47 |
1.3–2.2 |
* Relative risk, odds ratio or relative prevalence
Rates of NTM-PD are high in older individuals and those with underlying bronchiectasis.46,47 Many patients with bronchiectasis also have COPD (36–51%) or asthma (28–42%).23
In which patients should I rule out NTM?
NTM-PD should be ruled out in patients with underlying structural lung disease who:
- are being considered for long-term macrolide therapy to reduce exacerbations48
- are already receiving long-term macrolide therapy48
- present with worsening symptoms despite treatment optimisation49
- present with new pulmonary and non-specific systemic symptoms (e.g. chronic cough, fatigue, fever or dyspnoea).49
MAC-PD: difficult to treat
MAC-PD can be difficult to treat; MAC organisms evade host defences; they accumulate in biofilms and their uptake in macrophages gives them a place to hide from many antibiotics, which have poor penetration of these cells.50–54 Once inside macrophages, MAC limits normal macrophage function and reproduces unhindered, ready to trigger macrophage destruction so MAC bacteria can be released to infect the lung and invade other macrophages.55–57
Diagnostic criteria for MAC-PD
The 2020 international guidelines recommend that MAC-PD is diagnosed with either X-ray or computed tomography (CT) scan and the presence of MAC-positive sputum on multiple occasions. For radiological evidence of MAC-PD, either nodular or cavitary opacities on a chest radiograph, or bronchiectasis with multiple small nodules on a high-resolution CT scan is required. For microbiological confirmation, the 2020 guidelines recommend:5,19
- >1 positive sputum culture (to avoid spurious results from environmental contamination) with >3 respiratory samples collected over 1 week (to distinguish MAC-PD from occasional presence of MAC in the tracheobronchial tract).
- If sputum specimens are not obtainable, bronchoalveolar lavage fluid/bronchial washing cultures can be used to diagnose nodular/bronchiectatic NTM disease.
- Transbronchial or other lung biopsy with mycobacterial histologic features e.g., granulomatous inflammation or acid-fast bacilli (AFB) and positive culture for NTM or biopsy and one or more culture positive sputum or bronchial washings
Species identification helps determine clinical relevance and treatment selection.1,30 Where the same species is isolated in ≥2 sputum cultures over an interval of ≥1 week, there is a 98% likelihood of clinically significant MAC.
Following confirmation of a MAC-PD infection and the decision to treat, the next step is to test for antibiotic susceptibility5 to facilitate the selection of appropriate antimicrobial therapy.
Decision to treat MAC-PD
The decision to initiate antibiotic therapy for MAC-PD should not be based on diagnostic criteria alone.5,19 It is influenced by the severity of the disease, risk of disease progression, species/pathogenicity of infecting Mycobacterium, drug susceptibility, presence of comorbidity and the goals of treatment.1,5,19
Factors favouring treatment include those associated with poor prognosis (e.g. cavitary disease, low BMI, low albumin and elevated inflammatory markers), isolation of a species that is virulent and/or responsive to antimicrobial therapy, underlying immune suppression and major symptoms causing decreased health-related quality of life (e.g. fatigue).5,19 International NTM management guidelines recommend early treatment, as the benefits may outweigh the risks.5 Patients with NTM may already have a high treatment burden from their underlying chronic condition58–60 which could lead to a reluctance to add to it by starting treatment for NTM-PD immediately. However, with evidence from a study which suggests that left untreated, MAC-PD will progress,17 and increase risk of all-cause mortality27,61 and morbidity6–9,62 lowering patient quality of life,26,63 prompt treatment is essential.
Summary
Understanding which patients with underlying lung conditions are at risk of developing MAC-PD is important in order to deliver prompt and effective therapy. Treatment for MAC-PD is most successful at first initiation1 and a study has shown that refractory disease is associated with use of non-standard treatment regimens as outlined in the guidelines.64
Understanding the need to identify at-risk patients, implement effective diagnostic protocols and prompt robust therapy is a medical imperative with the potential to reduce patient mortality and morbidity.
Βιβλιογραφία:
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Eikhani MS, et al. BMC Infect Dis 2018;18:311.
- Maiga M, et al. PLoS One 2012;7:e36902.
- Wagner D, et al. Poster presented at: European Respiratory Society Annual Congress 6–10 September 2014; Munich, Germany. P1067.
- Daley CL, et al. Eur Respir J 2020b;56(1):2000535.
- Park HY, et al. Chest 2016;150:1222–32.
- Kobayashi T, et al. J Clin Tuberc Other Mycobact Dis 2018;11:17–21.
- Lee MR, et al. PLoS One 2013;8:e58214.
- Huang CT, et al. Int J Tuberc Lung Dis 2012;16:539–45.
- Marras TK, et al. Emerg Infect Dis 2017;23:468–76.
- Falkinham JO. J Appl Microbiol 2009;107:356–671.
- Falkinham JO. Clin Chest Med 2015;36:35–41.
- Nishiuchi Y, et al. Front Med 2017;4:27.
- Ratnatunga CN, et al. Front Immunol 2020;11:303.
- van Ingen J, et al. Int J Syst Evol Microbiol 2018;68:3666–77.
- Boyle DP, et al. Am J Respir Crit Care Med 2015;191:1310–17.
- Park TY, et al. PLoS One 2017;12:e0185774.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Daley CL, et al. Clin Infect Dis 2020a;71:e1–e36.
- Diel R, et al. BMC Infect Dis 2018;18:206.
- Terzikhan N, et al. Eur J Epidemiol 2016;31:785–92.
- Snell N, et al. Respir Med 2019;158:212–3.
- Chalmers JD, et al. Pulmonol 2018;24:120–31.
- Chalmers JD, et al. Chest 2018;165:1272–3.
- Taira N, et al. Am J Case Rep 2018;19:748–51.
- Yeung MY, et al. Respirology 2016;21:1015–25.
- Park CS, et al. Sci Rep 2019;9:15503.
- Kotilainen H, et al. Eur J Clin Microbiol Infect Dis 2015;34:1909–18.
- Liu C-J, et al. Respir Med 2019;151:19–26.
- Zweijpfenning SM, et al. Semin Respir Crit Care Med 2018;39:336–42.
- Aksamit TR, et al. Chest 2017;151:982–92.
- Andrejak C, et al. Thorax 2013;68:256–62.
- Jones MM, et al. PLoS One 2018;13:0197976.
- Dirac MA, et al. Am J Respir Crit Care Med 2012;186:684–91.
- Winthrop KL, et al. Ann Rheum Dis 2013;72:37–42.
- Brode SK, et al. Thorax 2015;70:677–82.
- Wu UI, et al. Lancet Infect Dis 2015;15:968–80.
- Szymanski EP, et al. Am J Respir Crit Care Med 2015;192:618–28.
- Kim RD, et al. Am J Respir Crit Care Med 2008;178:1066–74.
- Holt MR, et al. Eur Respir J 2019;54:1900252.
- Henkle E, et al. Clin Chest Med 2015;36:91–9.
- Ose N, et al. Surg Case Rep 2019;5:11.
- Friedman DZP, et al. Transpl Infect Dis 2020;22:e13229.
- Chao WC, et al. BMC Infect Dis 2017;17:796.
- Ku JH, et al. Diagn Microbiol Infect Dis 2020;96:114916.
- Prevots DR, et al. Am J Respir Crit Care Med 2010;182:970–6.
- Prevots DR, et al. Clin Chest Med 2015;36:13–34.
- Smith D, et al. BMJ Open 2020;7:e000489.
- Haworth C, et al. Thorax 2017;72:ii1–ii64.
- Awuh JA, et al. Cell Mol Life Sci 2017;74:1625–48.
- Ganbat D, et al. BMC Pulm Med 2016;16:19.
- Esteban J, et al. Front Microbiol 2018;8:2651.
- Chakraborty P, et al. Microbiol Cell 2019;6:105–22.
- McGarvey, et al. Clin Chest Med 2002;23:569–83.
- Chiplunkar SS, et al. Future Microbiol 2019;14:293–313.
- Gomes MS, et al. Infect Immun 1999;67:3199–206.
- Lee K-I, et al. Sci Rep 2016;6:37804.
- Global Burden of Disease 2015 Chronic Respiratory Disease Collaborators. Lancet Respir Med 2017;5:691–706.
- Lopez-Campos J, et al. Respirology 2016;21:14–23.
- Redondo M, et al. Breathe 2016;12:222–35.
- Diel R, et al. Eur Resp J 2017;49:1602109.
- Fleshner M, et al. Int J Tuberc Lung Dis 2016;20:582–7.
- Mehta M, et al. Respir Med 2011;105:1718–25.
- Fukushima K, et al. J Clin Med 2020;9:1315.
- Olivier KN, et al. Am J Respir Crit Care Med 2003;167:828–34.
- Roux A-L, et al. J Clin Microbiol 2009;47:4124–8.
- Hojo M, et al. Respirology 2012;17:185–90.
 Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Benefits of early treatment initiation in non-tuberculous mycobacterial pulmonary disease (NTM-PD)
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) with antimicrobial agents offers the possibility of cure.1 In patients who meet the clinical, radiographical and microbiological diagnostic criteria for NTM-PD, the 2020 ATS/ERS/ESCMID/IDSA clinical practice guideline for NTM-PD recommend initiation of treatment rather than watchful waiting.1 Initiation is especially important in the context of positive acid-fast bacilli sputum smears and/or cavitary lung disease1as there may be an increased rate of progression and poor treatment outcomes if treatment is delayed.1
Evidence of disease progression in untreated MAC-PD
Several studies have shown that most patients diagnosed with Mycobacterium avium complex pulmonary disease (MAC-PD) have progressive disease resulting in the need for antibiotic treatment.2,3 In a recent study of 488 newly diagnosed patients at the Asan Medical Center in South Korea, 305 (62.5%) patients showed progressive MAC-PD resulting in treatment initiation within 3 years of diagnosis.2 Similarly, in another study of 40 untreated patients with the nodular bronchiectatic form of MAC-PD (most with minimal symptoms), who underwent serial chest computed tomography (CT) scans for a minimum of 4 years, 39 (97.5%) experienced disease progression with a significant increase in overall CT score.3 It is noted in the 2020 NTM-PD guidelines that some subgroups (minimal nodular/bronchiectatic disease) may be safely, but regularly, followed without antimicrobial therapy; however, those with cavity disease should always receive prompt antibiotic treatment.1
Factors influencing the decision to initiate treatment
The decision to treat may be influenced by both host factors and infecting bacterial species. Certain factors like cavitary disease and low body mass index have been associated with progressive disease and may necessitate earlier consideration of antibiotic treatment.2 In very frail patients with very mild nodular bronchiectatic disease, the balance between efficacy and tolerability may favour watchful waiting.1
The clinical relevance of NTM varies significantly between species (Figure 1) and may also differ geographically.1,4 For example, species such as M. gordonae have low pathogenicity and rarely cause disease in humans, whereas M. kansasii is highly pathogenic.1,4
Figure 1. Clinical relevance (the percentage of patients with isolates of these species that meet the ATS/IDSA diagnostic criteria) of non-tuberculous mycobacterial species. M., Mycobacterium. Adapted from Zweijpfenning (2018).5

The most common NTM pathogens include MAC, M. kansasii and M. xenopi among the slowly growing NTM and M. abscessus among the rapidly growing NTM.1
Meeting the guideline-recommended diagnostic criteria for NTM-PD
Diagnostic criteria within the guideline is based on:
- Clinical symptoms e.g. worsening of symptoms of underlying lung conditions, or onset of new, persistent symptoms in patients at risk of NTM-PD e.g. haemoptysis, weight loss, fatigue
- Radiological findings on X-ray or hight-resolution CT scan such as nodular or cavitary opacities
- Microbiological findings from a) at least two expectorated sputum or b) positive culture from at least one bronchial wash or lavage or c) positive culture for NTM and biopsy from transbronchial or lung biopsy plus one or more culture positive sputa or bronchial washing.
Patients suspected of having NTM-PD who do not meet the diagnostic criteria should be actively managed and followed with serial CT scans until the diagnosis is firmly established or excluded and should start or continue recommended techniques such as airway clearance.6
The decision to initiate antibiotic treatment
NTM-PD is associated with diminished health-related quality of life that correlates with severity of lung impairment;7 antimicrobial treatment may be associated with improvement.8
NTM-PD treatment decisions are often difficult and require experience in managing the disease. This can mean that it may be necessary for a peer consultation or referral to a pulmonologist or infectious disease specialist with experience in NTM-PD.1,9 The virulence and potential for progressive disease must be evaluated once the NTM species is identified in order to determine treatment. In the 2020 ATS/ERS/ESCMID/IDSA clinical practice guideline for NTM-PD for example, it is recommended that for species of low pathogenicity such as M. gordonae, treatment is only indicated if repeated positive cultures over several months are observed, along with strong clinical and radiological evidence of disease whereas in many patients only one positive M. kansasii sputum culture may be required in order to initiate treatment.1 Similarly, clinically significant MAC-PD is unlikely in patients who have a single positive sputum culture during the initial evaluation but can be as high as 98% in those with ≥2 positive cultures.1 Two or more MAC-positive cultures indicate active MAC infection requiring a treatment decision, whereas for patients identified with M. kansasii, treatment should be initiated as soon as a single positive culture is obtained.1
Regardless of the infecting organism, the decision to initiate antibiotic treatment should be individualised considering the patient’s symptoms, the pathogenicity of the organism, radiological findings, microbiological results and importantly, the patient’s wish and ability to receive treatment as well as the goals of therapy.1 Any treatment decision should include a discussion with the patient that outlines the potential side-effects of antimicrobial therapy, the uncertainties surrounding the benefits of antimicrobial therapy and the potential for recurrence including reinfection (particularly in the setting of nodular/bronchiectatic disease).1 Guidelines recommend regular sputum cultures and routine monitoring to assess disease progression.1
 Following treatment initiation, sputum specimens should be obtained for culture every 1 to 2 months to document when sputum cultures become negative and to survey for the appearance of other organisms. 1
Following treatment initiation, sputum specimens should be obtained for culture every 1 to 2 months to document when sputum cultures become negative and to survey for the appearance of other organisms. 1
Clinical and radiographical assessments should be performed alongside the microbiological assessments to determine if the patient is responding to therapy.1
Retrospective studies have shown that most patients with MAC-PD who convert on treatment do so within 6 months of starting treatment.11–13
If you decide not to initiate antibiotic treatment, an active monitoring plan is recommended by the guidelines.1 Study data suggest that untreated NTM-PD could progress.2,3
Βιβλιογραφία:
- Daley Cl, et al. Clin Infect Dis 2020;71:e1–e36.
- Hwang JA, et al. Eur Respir J 2017;49:1600537.
- Park TY, et al. PLoS One 2017;12:e0185774.
- van Ingen J, et al. Thorax 2009;64:502–6.
- Zweijpfenning SMH, et al. Semin Respir Crit Care Med 2018;39:336–42.
- Lipman M, et al. BMJ Open Respir Res 2020 ;7 :e000591
- Mehta M, Marras TK. Respir Med 2011;105:1718–25.
- Czaja CA, et al. Ann Am Thorac Soc 2016;13:40–8.
- Ryu YJ, et al. Tuberc Respir Dis 2016;79:74–84.
- Lee MR, et al. Clin Microbiol Infect 2015;21:250.e1–250.e7.
- Furuuchi K, et al. Chest 2020;157:1442–5.
- Koh WJ, et al. Eur Respir J 2017;50:1602503.
- Moon SM, et al. Eur Respir J 2019;53;1801636.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
,
Επισκόπηση των κατευθυντήριων οδηγιών κλινικής πρακτικής από τους φορείς ATS/ERS/ESCMID/IDSA 2020 για τη θεραπευτική αντιμετώπιση της πνευμονικής νόσου από μη φυματικά μυκοβακτηρίδια
Non-tuberculous mycobacterial pulmonary disease (NTM-PD) can be life threatening and is increasing in prevalence. International guidelines updated in 2020 provide management recommendations for the four most commonly occurring NTM pathogenic species.
Non-tuberculous mycobacteria (NTM) are ubiquitous in the environment.1 The clinical presentation of NTM infection is most often pulmonary disease (PD), and rates are highest in elderly people, and in those with underlying structural airway disease, cancer or immunodeficiencies.1,2 Diagnosis and treatment of NTM-PD can be difficult2 and its prevalence is growing.1 Published in 2020, ATS/ERS/ESCMID/IDSA jointly sponsored the development of a guideline updating management recommendations for NTM-PD in adults.1 Recommendations for diagnosis and treatment of the most common pathogenic NTM species and the perspectives of international thought leaders on guideline recommendations are summarized here. The appearance of new guidelines in 2020 was widely welcomed by NTM experts.
“These guidelines represent an important achievement after 13 years with a rigorous, great methodology, starting from 22 PICO [Population, Intervention, Comparator and Outcome] questions addressed in these guidelines resulting in 31 recommendations touching the management of patients with NTM, including Mycobacterium avium complex, M. kansasii, M. xenopi or M. abscessus. The task force was composed of representatives from four different international societies, two from the US and two from Europe plus patient representatives, so a very important document in 2020”. Stefano Aliberti, University of Milan, Italy
Diagnosis
The ability of NTM to cause disease differs between species, with M. xenopi, M. kansassi, M. abscessus and Mycobacterium avium complex (MAC) of which M. avium and M. intracellulare are the most common species being responsible for most NTM-PD.1 It is clear from experts that awareness of NTM remains relatively low so understanding which patients might be at risk of NTM-PD can be suboptimal.
“Women are usually more affected than men and of course a number of patients with particular conditions like cystic fibrosis [and], or some kind of immunosuppression”. She went on to say that “It is difficult for many people to understand what patients should be tested for NTM, and when and for these you should be aware about compatible signs and symptoms. For example, persistent, long-term dry cough or over-productive cough is one of the most common symptoms, as is loss of weight, some mild fever at night and sweating”. Eva Polverino, Vall d’Hebron Hospital, Spain
Diagnosis relies on clinical, radiographical and microbiological data — laboratory identification to a species or subspecies level is key both in diagnosis and treatment decisions.1
The four most common pathogenic species are Mycobacterium avium complex (MAC), M. kansasii, M. xenopi and M. abscessus.1,3
- Clinical signs of NTM-PD include cough, sputum production, haemoptysis, dyspnoea chest pain, malaise, weight loss and night sweats.3
- Radiological signs are nodular or cavitary opacities on chest radiograph, or bronchiectasis with multiple small nodules and tree-in-bud pattern on high-resolution computed tomography scan.1
- Microbiological confirmation (one of the following) is obtained from:1
- positive culture results (same NTM species) from at least two sputum samples
- positive culture results from at least one bronchial wash or lavage
- transbronchial or other lung biopsy with mycobacterial histological features (granulomatous inflammation or acid-fast bacilli) and, either
- positive culture for NTM, or
- one or more sputum or bronchial washings culture positive for NTM.
Treatment
The recent guidelines recommend treatment initiation rather than watchful waiting on diagnosis of NTM-PD, but drug therapy should follow a careful discussion of risk–benefit with the patient.1 Treatment varies according to the species, extent of disease, drug susceptibility results and the patient’s underlying comorbidities.1 Regimens often require administration of multiple agents associated with clinically significant adverse events for a prolonged period, outcomes are often suboptimal and reinfection common. Expert consultation is often helpful.1
Guideline recommendations are made in several scenarios for patients infected with MAC, M. kansasii, M. xenopi or M. abscessus.1 However, before treatment is initiated understanding drug susceptibility for those drugs being considered for treatment is important.1
“The new guidelines do provide recommendations on drug susceptibility testing and they state that drug susceptibility testing should be performed before initiating treatment for MAC pulmonary disease for example. Importantly they state so for 2 drugs or groups of drugs, for macrolides and for amikacin. Testing amikacin susceptibility is very important because amikacin, as an IV drug, may be required in the initial phase of treatment in patients with very severe disease and in patients with refractory disease”. Jakko van Ingen, Radboud UMC, the Netherlands
Similarly, for M. abscessus susceptibility testing for macrolides and amikacin is also recommended, whilst for M. kansasii the guideline recommends susceptibility testing for rifampicin.1 For M. xenopi the guideline indicates there is insufficient evidence to recommend any specific susceptibility testing.1
MAC1
Macrolide-susceptible MAC-PD1
- A three-drug regimen including a macrolide is suggested in preference to a two-drug regimen that includes a macrolide and is recommended over a three-drug regimen with no macrolide.
- Macrolide susceptibility consistently predicts treatment success; regimens with no macrolide are associated with reduced rates of negative sputum-culture conversion, and with higher mortality.
- Three-drug regimens are recommended owing to a lack of evidence for the relative risk of macrolide-resistant MAC developing with two-drug versus three-drug regimens.
- In patients with non-cavitary nodular/bronchiectatic disease, administration of a macrolide-based regimen three times per week is preferable to daily administration.
- Intermittent and daily therapies have similar sputum conversion rates, but intermittent treatment is better tolerated and not known to be associated with development of macrolide resistance.
- In patients whose therapy has failed after at least 6 months of guideline-recommended oral guideline-based treatment, once-daily Amikacin Liposome Inhalation Suspension (ALIS) should be added to the treatment regimen.
- After culture conversion, patients should continue treatment for at least 12 months.
- This is based on the 2007 guideline recommendation (12 months of negative sputum cultures), and a lack of data on optimal duration of therapy.3
Newly diagnosed macrolide-susceptible MAC-PD1
- It is suggested that azithromycin-based, rather than clarithromycin-based, regimens are used and that initial treatment should not include inhaled amikacin (parenteral formulation) or ALIS.
- Clarithromycin and azithromycin have equal efficacy, but azithromycin is better tolerated, with fewer drug interactions, lower pill burden and is taken once daily.
Cavitary or advanced/severe bronchiectatic or macrolide-susceptible MAC-PD1
- A macrolide-based regimen administered daily rather than three times per week is suggested.
- No randomised trials have evaluated the the risk of macrolide resistance associated with intermittent versus daily regimens, so a daily regimen is preferred.
Cavitary or advanced/severe bronchiectatic or macrolide-resistant MAC-PD1
- Parenteral amikacin or streptomycin is suggested as part of initial treatment.
- Parenteral aminoglycoside is among very few options for ‘intensifying’ standard oral therapy in MAC. Administration of aminoglycoside for at least 2–3 months balances associated risks and benefits.
M. kansasii1
Rifampicin-susceptible M. kansasii-PD1
- A regimen of rifampicin, ethambutol and either isoniazid or a macrolide is suggested instead of a fluoroquinolone. It is also suggested that neither parenteral amikacin nor streptomycin be used routinely. Patients should be treated for at least 12 months.
- Isoniazid is widely used with rifampicin and ethambutol, with good outcomes. Two studies substituting isoniazid with clarithromycin also showed good treatment outcomes. There is less experience for substitution with a fluoroquinolone, but this can be used in cases of antibiotic intolerance or rifampicin resistance.
- Apart from in severe disease or where rifampicin-based regimens cannot be used, the risk of adverse reactions counts against parenteral amikacin or streptomycin.
- Daily administration is suggested for all patients receiving rifampicin, ethambutol and either isoniazid or a macrolide.
- Patients with non-cavitary nodular/bronchiectatic disease receiving the macrolide-containing regimen can also be treated three times weekly.
M. xenopi1
All patients1
- A daily treatment regimen of at least three drugs is suggested: rifampicin, ethambutol and either a macrolide and/or a fluoroquinolone (e.g. moxifloxacin). It is suggested that treatment continues for at least 12 months after culture conversion.
- In cavitary or advanced/severe bronchiectatic disease, adding parenteral amikacin to the treatment regimen and obtaining expert consultation are suggested.
- On current evidence, patients should be treated aggressively given the high mortality associated with xenopi-PD.
M. abscessus1
All patients1
- A regimen of at least three drugs is suggested (selection guided by in vitro susceptibility)
- The disease can be life threatening. Treatment regimens should be designed with expert guidance in particular with respect to treatment duration.
Strains without inducible or mutational resistance1
- A macrolide-containing multidrug treatment regimen is recommended.
- In vitro macrolide-susceptibility testing is important.
Strains with inducible or mutational macrolide resistance1
- It is suggested that a macrolide can be included for its immunomodulatory properties but is not considered part of the antibiotic regimen.
“In addition to antibiotic therapy close monitoring of the patient and patient education is very important. One of the major mistakes done in the management of patients with Mycobacterium avium pulmonary disease or any other NTM pulmonary disease, is the lack of proper monitoring, for example monthly sputum collection for microscopy culture. Also the weight of a patient and symptoms, systems recording like sputum production or fatigue recording are important as part of the monitoring of a patient during the treatment”. Christoph Lange, Research Center Borstel, Germany
The guidelines published in 2020 are very welcome in this often overlooked area of respiratory medicine. However, it is clear that treatment for patients with NTM-PD remains arduous and lengthy with multiple drug regimens that are required for 12 months or more post-culture conversion.
Abbreviations
ALIS, Amikacin Liposomal Inhalation Solution; ATS, American Thoracic Society; ERS, European Respiratory Society; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; IDSA, Infectious Diseases Society of America; MAC, Mycobacterium avium complex; NTM, Non-tuberculous mycobacteria; NTM-PD, Non-tuberculous mycobacterial pulmonary disease; PD, pulmonary disease.
Βιβλιογραφία:
- Daley CL, et al. Eur Respir J 2020;56(1):2000535.
- Ratnatunga CN, et al. Front Immunol 2020;11:303.
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Understanding best practice in Mycobacterium avium complex pulmonary disease (MAC-PD)
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) varies depending on the species, extent of disease, drug susceptibility results and underlying comorbidities.1 Mycobacterium avium complex (MAC) is the most common cause of disease and a multidrug antimicrobial regimen is recommended, first line, to avoid the development of resistance.1,2 Treatment is lengthy and adverse events are not uncommon; successful treatment requires good adherence, frequent monitoring and effective adverse event management.1,3
Initiation of treatment for MAC-PD
Treatment of MAC-PD with antimicrobial agents offers the possibility of cure of the disease.1 In patients who meet the diagnostic criteria (clinical symptoms, radiological evidence of nodules, bronchiectasis or cavitary opacity, and microbiological evidence of positive culture results from sputum), the ATS/ERS/ESCMID/IDSA 2020 NTM guidelines suggest initiation of treatment rather than watchful waiting, especially in the context of positive acid-fast bacilli sputum smears and/or cavitary lung disease.1 The decision to initiate antimicrobial therapy should nonetheless be individualised and based on a combination of clinical factors, the infecting species and individual patient priorities.1,4
Comprehensive care and good communication across a multidisciplinary team of healthcare providers is important because of the complexities of MAC-PD management and treatment.5–7 When initiating treatment, setting expectations for the patient is critical and it is important to discuss length of therapy, treatment response, follow-up appointments and potential adverse events.1
“One needs to discuss with the patient the likely side-effects of treatment, the likely success or failure of treatment and, of course, the potential for reinfection.” Charles Haworth, Royal Papworth Hospital, UK
“If you need to start treatment that you’re inexperienced with or you run into toxicity or other issues that you’re inexperienced in, consult centres of excellence in this field to improve management of the patient.” Jakko van Ingen, Radboud UMC, the Netherlands
Drug susceptibility testing for MAC-PD
Macrolide monotherapy is commonly prescribed in NTM-PD and can be a key driver in the development of macrolide-resistant strains, leading to poor outcomes for NTM treatment.8
Given the good correlation between in vitro activity and in vivo outcomes with macrolides9 and amikacin3 for MAC, the 2020 NTM guidelines recommend susceptibility-based treatment for macrolides and amikacin over empirical therapy for patients with MAC-PD.1 Recommendations, including protocols and related quality-control parameters, for the susceptibility testing of mycobacteria are provided by the Clinical and Laboratory Standards Institute (CLSI).10
“Drug susceptibility testing should be performed for the macrolides and amikacin before the onset of treatment.” Jakko van Ingen, Radboud UMC, the Netherlands
“For patients with MAC lung disease, it’s essential to perform drug susceptibility testing on isolates before you commence treatment. In particular, it’s crucial to know the macrolide susceptibility as that will influence the initial regimen and it’s also important to know the amikacin susceptibility, particularly in patients with severe disease or cavitary disease.” Charles Haworth, Royal Papworth Hospital, UK
Multidrug treatment regimen for MAC-PD
Patients respond best to MAC treatment regimens the first time they are administered; therefore, it is especially important that patients receive recommended multidrug therapy the first time they are treated for MAC-PD, especially as up to 45% of patients are known to fail first-line therapy.11–14
Macrolides (clarithromycin and azithromycin) are a key component of MAC-PD treatment based on data that shows poor patient outcomes if they are excluded.1 The 2020 NTM guidelines recommend treatment with at least three antimicrobials (Table 1) for macrolide-sensitive MAC-PD with the addition of parenteral amikacin or streptomycin in macrolide-insensitive disease or advanced bronchiectatic/cavitary disease.1
Macrolide resistance is associated with higher mortality rates than macrolide-sensitive disease, with one study suggesting a mortality rate of >45% over 5 years.8 Treating macrolide-resistant disease can be difficult and expert consultation should be sought.1
“The most important thing in treating macrolide-resistant MAC pulmonary disease is to look for help… seek expert consultation.” Jakko van Ingen, Radboud UMC, the Netherlands
Table 1. Guideline-based therapy for Mycobacterium avium complex pulmonary disease (MAC-PD).1
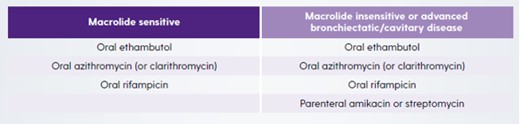
For both macrolide-sensitive and macrolide-insensitive disease, azithromycin is recommended over clarithromycin because of better tolerance, fewer drug interactions, lower pill burden and equal efficacy.1 However, when azithromycin is not available or not tolerated, clarithromycin is considered an acceptable alternative.1 Ethambutol is included in the recommendation as it is the most effective drug known to prevent the development of macrolide resistance.15,16
“The three-drug regimen should be based on azithromycin as the macrolide of choice, plus ethambutol as a second drug and a third companion drug, most likely being a rifamycin. In the case of severe disease, like fibro-cavitary disease or disease that affects both lungs, additional treatment with a parenteral aminoglycoside like streptomycin or amikacin should be considered.” Christoph Lange, Research Center Borstel, Germany
“The guidelines are very clear that azithromycin is preferable over clarithromycin in most circumstances and that’s because, on the whole, it’s better tolerated particularly from a gastrointestinal perspective, it’s once a day rather than twice a day and there are fewer drug–drug interactions, particularly with rifampicin.” Charles Haworth, Royal Papworth Hospital, UK
In patients with non-cavitary nodular/bronchiectatic disease a dosing regimen of three times per week is recommended but in patients with cavitary severe/advanced disease, treatment should be administered daily.1
Monitoring for treatment response in MAC-PD
The 2020 NTM guidelines recommend frequent follow-up visits after initiating treatment for MAC-PD, including obtaining sputum cultures every 1 to 2 months to determine if, and when, culture conversion occurs.1 Retrospective studies have shown that among the NTM-PD patients who convert on standard first-line multidrug treatment, the majority do so within 6 months of treatment initiation.17–19 In addition to microbiological assessments, clinical and radiographical findings should also be used to determine if the patient is responding to therapy.1
“One of the major mistakes done in the management of patients with Mycobacterium avium complex pulmonary disease or any other NTM pulmonary disease, is the lack of proper monitoring, for example monthly sputum collection for microscopy and culture.” Christoph Lange, Research Center Borstel, Germany
If patients do not respond as expected, therapeutic drug monitoring could be considered in situations where drug malabsorption, drug underdosing or clinically important drug–drug interactions are suspected.20
It is recommended that treatment should be maintained for at least 12 months after culture conversion to increase chances of treatment success.1 Of note, in a study of 154 patients with MAC-PD, those who were treated for <15 months after culture conversion were twice as likely to experience recurrence than those treated for ≥15 months post conversion.17
Management of patients with MAC-PD who fail to culture convert
Study data has shown that up to 45% of MAC-PD patients will fail to respond on standard first-line multidrug treatment.13,14 Non-conversion may be an early sign that the patient may have future radiographic progression and lung function decline.21,22
In cases of MAC-PD where sputum cultures do not convert after 6 months of treatment, the 2020 NTM guidelines recommend that Amikacin Liposomal Inhalation Suspension (ALIS) once daily is added to the regimen.1
“When mycobacterial cultures do not convert by 6 months on oral guideline-based therapy patients should be considered to receive amikacin liposomal inhalation suspension. This has been shown to increase the chances to achieve culture conversion by 12 months and the effect of culture conversion is sustained for at least 3 months past the end of therapy.” Christoph Lange, Research Center Borstel, Germany
In selected patients with failure of medical management, cavitary disease, drug-resistant isolates or complications such as severe bronchiectasis, surgical resection of the diseased lung may be appropriate. The risks and benefits of surgery should be weighed up and expert consultation sought.1
“Surgical resection of the most affected areas of the lung can help to achieve cure in patients.” Jakko van Ingen, Radboud UMC, the Netherlands
“In patients with macrolide-resistant MAC-PD, we should also consider the role of surgery. These patients should be discussed with an expert surgeon because in some cases surgery could be on top of treatment and could improve outcomes for patients with macrolide-resistant MAC-PD.” Stefano Aliberti, University of Milan, Italy
Monitoring for adverse reactions in treatment of MAC-PD
The drugs routinely used to treat MAC-PD are frequently associated with adverse reactions,1 as demonstrated in a recent randomised clinical trial where >90% of participants experienced an adverse event.3 Consequently, educating patients regarding potential adverse reactions and monitoring them are important components of patient management. Furthermore, rapid identification and management of an adverse reaction may decrease the risk of treatment discontinuation and possibly improve the chances of treatment completion.1 Where drug intolerance is suspected, some medications could be introduced gradually at 1- to 2-week intervals so that appropriate evaluations of tolerance can be performed.11 According to the ATS/ERS/ESCMID/IDSA 2020 guidelines, it is important to individualise the frequency of monitoring for adverse reactions based on patient age, comorbidities, concurrent drugs, overlapping drug toxicities and resources.1 It is recommended that patients should have a complete blood count, liver function tests and metabolic panel every 1–3 months in patients on oral therapy and weekly if on intravenous therapy.1 Depending on the antibiotics selected, there may be a need to refer to other specialists for routine monitoring, including an ophthalmologist for vision testing and an audiologist to take baseline audiograms and hearing tests (Table 2).1
“Blood monitoring is essential because many of the treatments are quite toxic, so you’ll do full blood counts to look for bone marrow toxicity, liver blood tests and renal monitoring…Depending on the regimen you may want to do an ECG, to check for QT prolongation and often we’ll do audiology in patients that are on azithromycin or amikacin, to look for evidence of hearing impairment.” Charles Haworth, Royal Papworth Hospital, UK
Table 2. Common adverse reactions associated with drugs used to treat Mycobacterium avium complex pulmonary disease (MAC-PD) and monitoring recommendations.1
|
Drug |
Adverse Reactions |
Monitoring |
|
Azithromycin/clarithromycin |
Gastrointestinal Tinnitus/hearing loss Hepatotoxicity Prolonged QTc |
Clinical monitoring Audiogram Liver function tests ECG (QTc) |
|
Ethambutol |
Ocular toxicity
Neuropathy |
Visual acuity and colour discrimination Clinical monitoring |
|
Rifampicin |
Hepatotoxicity Cytopenias Hypersensitivity Orange discoloration of secretions |
Liver function test Complete blood count Clinical monitoring |
|
IV Amikacin/Streptomycin |
Vestibular toxicity Ototoxicity Nephrotoxicity Electrolyte disturbances |
Clinical monitoring Audiograms BUN, creatinine Calcium, magnesium, potassium |
BUN, blood, urea, nitrogen; ECG, electrocardiogram.
Best practice beyond pharmacotherapy alone
Providing best practice in MAC-PD requires an holistic approach to patients that explores non-pharmacological interventions as well as medication regimens outlined in the ATS/ERS/ESCMID/IDSA 2020 guidelines.
From the point of diagnosis, patients should be encouraged to undertake airway clearance techniques in order to limit lung function decline.23 The patient journey for MAC-PD patients is long, and many will have lived with their condition for some time before diagnosis.24 Living with a chronic, potentially debilitating disease is hard for patients and has a negative impact on their quality of live. Psychological interventions may be relevant for these patients and should be considered. Similarly, it is known that low body mass index is a risk factor for NTM-PD and is often a clinical characteristic of these patients. In these patients, nutritional support is important and involving a nutritionist or dietician in the clinical team may be helpful as improving diet and stopping weight loss can help to counter low mood.23
Summary
Patients with MAC-PD often face a long and punishing treatment journey with lengthy and complex multidrug regimens, many of which are associated with adverse events that affect patient adherence. Best practice management for MAC-PD should consider all aspects of the patient’s journey from non-pharmacological interventions, such as airway clearance, through to collaboration with other specialists for example radiologists, respiratory physicians, psychologists, dieticians, specialist pharmacists, nurses and physiotherapists to optimise patient’s nutrition, sleep and mental state.
Βιβλιογραφία:
- Daley CL, et al. Eur Respir J 2020;56(1):2000535.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Griffith DE, et al. Am J Respir Crit Care Med 2018;198:1559–69.
- Haworth C, et al. Thorax 2017;72:iii1-ii64.
- Ryu YJ, et al. Tuberc Respir Dis (Seoul) 2016;79:74–84.
- Yu JA, et al. Thorac Surg Clin 2012;22:277–85.
- van Ingen J. Semin Respir Crit Care Med 2013;34:103–9.
- Moon SM, et al. Antimicrob Agents Chemother 2016;60:6758–65.
- Kobashi Y, et al. J Infect Chemother 2006;12:195–202.
- Clinical and Laboratory Standards Institute. M48 - laboratory detection and identification of mycobacteria, 2nd edition 2018Clinical and Laboratory Standards Institute. M24 – Susceptibility Testing of Mycobacteria, Nocardia spp, and other Aerobic Actinomyces, 3rd edn. 2018.
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Griffith DE, Aksamit TR. Curr Opin Infect Dis 2012;25:218–27.
- Fukushima K, et al. J Clin Med 2020;9:1315.
- Wallace RJ, et al. Chest 2014;146:276–82.
- Griffith DE, et al. Am J Respir Crit Care Med 2006;174:928–34.
- Morimoto K, et al. Ann Am Thorac Soc 2016;13:1904–11.
- Furuuchi K, et al. Chest 2020;157:1442–5.
- Koh WJ, et al. Eur Respir J 2017;50:1602503.
- Moon SM, et al. Eur Respir J 2019;53:1801636.
- Nahid P, et al. Clin Infect Dis 2016;63:e147–95.
- Park HY, et al. Chest 2016;150:1222–32.
- Pan SW, et al. Clin Infect Dis 2017;65:927–34.
- Lipman M, et al. BMJ Open Resp Res 2020;7:e000591.
- Kotilainen H, et al. Eur J Clin Microbiol Infect Dis 2015;34:1909–18.
 Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.